Cucurbit Genetics Cooperative Report 6:73-74 (article 37) 1983
J. E. Ferguson, D. C. Fischer, and R. L. Metcalf
Departments of Entomology and Horticulture, University of Illinois, Urbana-Champaign, IL 61801
The tetracyclic triterpenoid cucurbitacins, bitter substances of the Cucurbitaceae, are highly toxic to mammals with intraperitoneal median lethal dose values for pure Cucs in the mouse of 1.2 mg Cuc A/kg; 1.0 mg Cuc B/kg; 6.8 mg Cuc C/kg and in the rat 2.0 mg Cuc A/kg (1). Cases of stock poisoning, especially in times of drought, have been recorded in South Africa after feeding on Cucumis leptodermis Schwerch, C. africanus L., and C. myriocarpus Naud. (2).
Cucurbitacins have been bred out of cultivated fruit but are typically present in large amounts in wild cucurbits. This laboratory, however, was recently consulted about a number of cases of human poisoning over the period of a year from the consumption of commercially produced zucchini (Cucurbita pepo L.) in Australia. Characteristic symptoms included a bitter taste in the mouth, stomach cramps, diarrhea and occasionally collapse (Mr. M. Herrington, Horticulturist Redlands Horticultural Research Station, Queensland, Australia, personal communication). In one case after five people consumed approximately 700 g each of zucchini within a 6 hour period, two people collapsed and the others suffered severe cramps and diarrhea. The consumption of bitter tasting zucchini prior to the onset of symptoms implicated cucurbitacins.
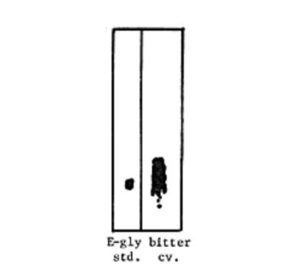
Analysis of a freeze-dried sample of very bitter zucchini provided to us by the Redlands Horticultural Research Station determined beyond question that cucurbitacins were present. A well established bioassay technique using TLC and Diabroticite beetles showed an Rf value corresponding to Cucurbitacin E-glycoside (Figure 1) (3). The identity of the Cuc was qualitatively verified by high pressure liquid chromatography (HPLC) using a Waters model M-45 single pump system with a radially compressed octadecyl silane column with a solvent of 73% methanol and a flow rate of 1 ml/min. The five major cucurbitacins of Cucurbita-B, D, E, I and E-glycoside can be separated by HPLC (4). Standard Cuc E-glycoside extracted from Cucurbita texana and verified by mass spectrometry, was co-injected with the sample and retention times were identical. Quantitative determination of the Cuc content by UV absorption spectrometry at 210 nm revealed 1.12 mg Cuc E-glycoside/g fresh wt. fruit (if one assumes a loss of 90% moisture with drying). This remarkably high level of cucurbitacin is comparable to that of many wild cucurbits and undoubtedly could account not only for the bitter taste of the zucchini cultivar but the symptoms of poisoning reported in Australia.
So, far, based on traces back to grower’s fields, only one cultivar, a widely grown hybrid zucchini, has been implicated. In the one instance where the culprit plant producing the bitter fruit was identified, the plant’s leaves showed more silvering, the fruits were shorter, broader and somewhat lighter in color, and the mature fruit was yellow and somewhat warty in contrast to the normal black green smooth rind. Further studies of the basis for Cuc synthesis in the zucchini are currently in progress with seed from this plant.
Literature Cited
- David, A. and D. K. Vallance. 1955. J. Pharm. Pharmacol. 7:295–296.
- Watt, J. M. and M. G. Breyer-Brandwijk. 1962. The Medicinal and Poisonous Plants of Southern and Eastern Africa. 2nd ed., E. & S. Livingstone. Edinborough. 1457 pp.
- Metcalf, R. L., A. M. Rhodes, R. A. Metcalf, J. E. Ferguson, E. R. Metcalf, and P. Y. Lu. 1982. Environ. Entomol. 11:931–927.
- Ferguson, J. E., E. R. Metcalf and R. L. Metcalf. 1983. J. Econ. Entomol. (in press).
This research was supported in part by a grant from USDA, SEA Competitive Research Grants Office Ag 81-CRCR-1-0659. Any opinions, findings and conclusions are those of the authors and do not necessarily reflect the views of USDA.