Cucurbit Genetics Cooperative Report 7:55-57 (article 25) 1984
Thomas, Claude E.
U.S. Department of Agriculture, Agricultural Research Service, U.S. Vegetable Laboratory, 2875 Savannah Highway, Charleston, SC 29407
Alternaria cucumerina incites leaf blight of various cucurbits throughout the world (2). This disease is particularly severe on muskmelon (Cucumis melo L.) in the southeastern and Midwestern production areas of the U.S.A. Studies by the author into disease control (4) and epidemiology of Alternaria leaf blight have developed information of use to those interested in the evaluation of muskmelon germplasm for resistance to this disease. The objectives of the studies reported herein were to develop practical inoculation procedures with A. cucumerina based on inoculum concentration, temperature, and duration of leaf wetness.
A. cucumerina was grown on V-8 juice agar under fluorescent illumination in an alternating regime of 8 hr light- 16 hr dark. Spores were harvested from 10-14-day-old cultures by flooding the surface of the media with sterile distilled water and scraping with a large glass coverslip to detach the conidia. The resultant suspension was then thoroughly mixed and clumps were dispersed by a 15-second treatment in the microcup of a Waring blender at high speed. The suspension was then diluted to the desired concentration. It was kept agitated to prevent settling of the conidia during this procedure.
Conidial suspensions were sprayed onto leaves using a DeVilbiss No. 15 atomizer or a Paasche Type H airbrush. The first two expanded leaves of ten 21-day-old ‘Perlita’ plants were sprayed to run-off and the plants were then placed in a high humidity chamber at the desired temperature for the duration of the leaf wetness period. At the end of the leaf wetness period, plants were removed and placed under fluorescent, supplemented by incandescent, illumination for 12-hr photoperiods at 20 C. The sixth night after inoculation, plants were placed back in the high humidity chamber for 16 hr at 20% to induce sporulation, which was observed but not evaluated in these studies. This high humidity treatment also makes severely diseased and desiccated leaves easier to handle during the evaluation process. Plants were removed from the chamber and determinations of lesion size, number, and type were made on the seventh day. All tests were repeated at least twice.
Inoculum concentration study. Inoculations were made with inoculum concentrations (conidia/ml) of: 0.5 x 103, 1.0 x 103, 2.0 x 103, 3.0 x 103, 4.0 x 103, 5.0 x 103, 6.0 x 103, 7.0 x 103, 8.0 x 103, 9.0 x 103, 1.0 x 104, 2.5 x 104, 5.0 x 104, 7.5 x 104 and zero (noninoculated check). Inoculated and check plants were then placed at high humidity for 16 hr at 20%. At inoculum concentrations of 2.5 x 104 and higher, many inoculated leaves were dead and desiccated by the sixth day. Lesions on those which were still alive were so numerous and coalesced that accurate determinations of number and size were extremely difficult. At 1.0 x 104 numerous coalesced lesions also precluded accurate determinations. As inoculum levels decreased, the number of coalesced lesions decreased, so that at 5.0 x 103 conidia/ml and lower, almost all lesions were discrete. At concentrations below 5.0 x 103, however, leaves without lesions (escapes) were encountered with increasing frequency as inoculum levels decreased. At 0.5 x 103 about 25% of the inoculated leaves escaped infection. Therefore, for subsequent studies, an inoculum concentration of 5.0 x 103 was used since it gave the most discrete lesions with minimal coalesced lesions without escapes. Carmody et al. (1) reports an optimum conidia concentration of 3.0 x 103 at 27C for leaf infection. Norton and Boyhan (3) used 2.0 x 104 at 25 C. Since these researchers were using the same A. cucumerina isolate (furnished by C.E. Thomas) in their work, the differences in inoculum concentrations are probably due to differences in temperature, duration of high humidity period, and plant age.
Temperature study. Ten plants were inoculated as above and were immediately placed under high humidity in dark chambers at either 5, 10, 15, 20, 25, 30 or 35 C for 16 hr. Only a trace amount of infection occurred at either 5 C or 35 C. Infection was highest at 10 C and declined as temperature increased. A temperature of 20 C was chosen for use in subsequent studies. This temperature represents the lowest night temperature that one would reasonably expect to encounter with any frequency during a muskmelon growing season in areas where this disease occurs.
Leaf wetness study. Plants were inoculated as above and placed in the dark at high humidity at 20 C. Ten plants were removed from the high humidity chamber at 2 hr intervals from 2- 24 hr. The results of duration of leaf wetness studies (Figure 1) are given as totals for 20 leaves at each wetness treatment. There was no lesion development from the 2 hr treatment. Less than one lesion/leaf developed from the 4, 6, and 8 hr leaf wetness durations. Significant lesion development did not occur until 10 hr, and the number of successful infections increased sharply as the hours of leaf wetness increased. However, after 16 hr, the increased level of successful infections resulted in increased levels of coalesced lesions which made accurate comparisons difficult without careful examination of these lesions at magnifications of 25-50 x. Since this would not be conducive to a rapid evaluation technique, 16 hr of leaf wetness is recommended as the maximum duration.
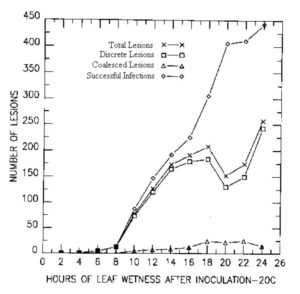
Figure 1. Effect of duration of leaf wetness period on lesion development on C. melo by A. cucumerina at 20 C.
In summary, the recommended inoculation conditions for evaluation of C. melo against A. cucumerina at the two expanded leaf stage are: inoculum concentration of 5.0 x 103, 20 C, and 16 hr duration of leaf wetness in the dark. After this inoculation treatment, plants may be kept at constant temperatures or at usual greenhouse temperatures until they are evaluated. If sporulation is to be rated, a uniform 20 C, 16 hr leaf wetness, dark treatment should be provided on the sixth night after inoculation prior to evaluations on the seventh day.
Literature Cited
- Carmody, B.E., M.E. Miller and M.P. Crisham. 1983. A technique for screening cantaloup seedlings for Alternaria cucumerina resistance. Phytopathology 73:499. (Abstract)
- Jackson, C.R. 1959. Symptoms and host-parasite relations of the Alternaria leaf spot disease of cucurbits. Phytopathology 49:731-733.
- Norton, J.D. and G.E. Boyhan. 1983. Resistance to Alternaria cucumerina in muskmelon. HortScience 18:602. (Abstract)
- Thomas, C.E. 1983. Fungicide applications based on duration of leaf wetness periods to control Alternaria leaf blight of cantaloup in south Texas. Plant Dis. Reptr. 67:145-147.
This research was supported by Grant No. US-287-81 from the United States-Israel Binational Agricultural Research and Development (BARD) Fund.