Cucurbit Genetics Cooperative Report 8:82-84 (Article 32) 1985
Nijs, A. P. M. den, C. J. M. Denissen and M. A. P. F. Hornes
Institute for Horticultural Plant Breeding (IVT), P. O. Box 16, 6700 AA Wageningen, The Netherlands
The technique of in vivo egg cell transformation (3) requires that following a high dose irradiation (1 kGy and higher) pollen of the donor species is still vital enough to penetrate the embryo sac and deposit (some of) its DNA content into the egg cell. Additionally, development of endosperm is needed to ensure the initial survival of the pathenogenetic or (partly) hybrid embryo. At the IVT we are evaluating the above technique in order to introduce into the cucumber valuable resistances, which occur in non-crossable Cucumis species.
As a first step, in vitro pollen germination was examined of nine wild Cucumis species in relation to irradiation dose to assess their potential as donor species for in vivo egg cell transformation of the cucumber. Pollen germination and pollen tube growth in vitro gave a reasonable indication of the capacity of irradiated pollen to germinate and penetrate in vivo the style and ovules of C. sativus (1). The accessions used are listed below with their IVT-Genebank number:
| la C. ficifolius 2x | 1801 | 5 C. africanus | 1788 |
| lb C. ficifolius 4x | 1729 | 6 C. metuliferus | 1775 |
| 2a C. anguria | 0307 | 7 C. dipsaceus | 1728 |
| 2b C. anguria var. longipes | 1735 | 8 C. melo var. agrestis | 2160 |
| 3a C. zeyheri 2x | 0181 | 9 C. meeusii | 1800 |
| 3b C. zeyheri 4x | 1807 | 10 C. figarei | 1804 |
| 4 C. sativus var. hardwickii | 0777 | 11 C. myriocarpus | 0202 |
Male flowers of these accessions were irradiated by a cobalt source with 0, 1, 2, 3 or 4 kGy (1 Gy = 100 rad). Soon afterwards four pollen samples per treatment were germinated in vitro during 2 hours at room temperature and 100% RH in the light. Three hundred pollen grains were counted per treatment to calculate the germination percentage. The length of the pollen tubes of 60 germinated grains was measured to determine the average tube length. All treatments were carried out twice, in June and July 1984. Data were analysed by a multivariate analysis of variance (2). Quadratic dose-response functions for germination percentage and pollen tube length were constructed with parameters N (level), L (slope) and Q (curvature). The L-values are the best indication of the resistance of the pollen to an increasing irradiation dose. These L-values are negative because a higher irradiation dose normally leads to a diminished germination and pollen tube growth.
The L-values for the germination percentage (L1) were significant for all but one species, C. melo var. agrestis. Only a few L-values for pollen tube length (L2) deviated significantly from zero, probably because of large, nonsystematic differences between the two sampling dates. this awaits further analysis. Both accessions of C. anguria were very sensitive to irradiation. By contrast, C. figarei was hardly affected. The L1– and L2-values were not significantly correlated (r = 0.32)1.
Correlations were calculated between the L-values and the total amount of DNA per nucleus per species as determined in interphase cells by Ramachandran (4). These values are not always of the same accession, but variation within a species appeared to be small (4).
L1 for 11 species was not correlated with DNA amount (r = 0.36), but the correlation between L2 and DNA amount was appreciable (r = 0.60). With 9 degrees of freedom this r is just not sifnificant at 5%, but significant at 10%. Therefore about one third of the variation amongst the species with respect to resistance of pollen tube growth against irradiation is explained by variation in the amount of DNA per nucleus, the species with a higher amount being more resistant (see Figure 1).
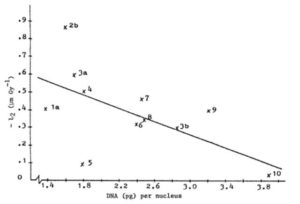
Figure 1. Relation between DNA amount per nucleus and sensitivity of pollen tube growth to irradiation of 11 Cucumis species
Literature Cited
- Boom, J. M. A. van den, and A. P. M. den Nijs. 1983. Effects of gammaradiation on vitality and competitive ability of Cucumis pollen. Euphytica 32:677-684.
- Keuls, M. and F. Garretsen. 1982. Statistical analysis of growth curves in plant breeding. Euphytica 31:51-64.
- Pandey, K. K. 1975. Sexual transfer of specific genes without gametic fusion. Nature 256:310-313.
- Ramachandran, C. 1984. Nuclear DNA variation in Cucumis species. Cucurbit Genetics Coop. Rpt. 7:97-99.