Cucurbit Genetics Cooperative Report 14:74-77 (article 28) 1991
R.Santana, L. A. Roig and V. Moreno
Plant Cell & Tissue Culture Laboratory, Biotechnology Department, Universidad Politecnica de Valencia, Cno. de Vera, 14 46020-Valencia (Spain)
Introduction. cucumis myriocarpus is a cucurbitaceous wild species in which resistances to Tetranychus urticae, CGMMV, powdery mildew ((PM1), and yellowing disease hve been reported (1, 2, 3, 4, 5). It is, therefore, an ideal partner for use in experimens of somatic hybridization with cultivated species of Cucumis with the aim of introgressing those resistance carrying genes into their genetic context. In order to approach these hybridizations efficiently the availability of good selection methods for the hybrid cells after fusion of protoplasts would be desirable. Agrobacterium-mediated transformation offers a rapid and efficient method for the introduction of antibiotic-resistance genes carried in engineered Ti plasmids into the plants regenerated from callus grown after co-cultivation of primary explants with the bacteria. These transgenic plants are particularly useful biochemical markers for protoplast fusion experiments, so it is very important to develop efficient and reproducable methods for plant regeneration at high rates from primary callus of this wild species.
A considerable handicap in undertaking studies on morphogenesis in explant-derived callus with several interesting accessions of this wild species is related to their very low rate of in vitro seed germination under the photoperiod conditions normally used by us with other cucurbitaceous species (6, 7). Studies carried out in our laboratory in order to improve the germination rate have indicated that short periods of seed incubation under darkness and higher temperature dramatically increase the percentage of germination.
In this paper, the results of this study and the preliminary results of a series of studies aimed at establishing the best cultural conditions leading to the attainment of plant regeneration from explant-derived callus of this wild species, are described.
Material & Methods. Seeds pf Cucumis myriocarpus L-2 (accession CuC 31/1983, kindly supplied by Dr. Chr. Lehmann, Zentralinstitut fur Genetik und Kulturpflanzen-forschung, Gatersleben, DDR), were decoated, surface sterilized by immersion in 30% commercial bleach (50 g/l active chlorine) for 30 min. followed by three rinses with sterile distilled waer and germinated on MG medium (6), either under 16 h photoperiod (1300 lux, Gro-lux, Sylvania) at 27+2 ˚ C day/24+2 ˚ C night or under continuous darkness at 32 ˚ C.
Explant sources for the experiments on morphogenesis were cotyledons taken off from seedlings at the stage of first true leaf apparition (5-7 days-old). This stage was accomplished under four treatment conditions: seed germination and growth of seedling always under photoperiod (D-1 seedling treatment condition), seed germination in darkness until plumule apparition and transferto photoperiod for seedling growth (D-2 condition), seed germination and growth until hypocotyl development around 1 cm length in the dark and the remaining growth under photoperiod (D-3 condition), and seed germination and growth in continuous darkness (D-4 condition).
Cotyledon explants were obtained as previously described for melon (7) and placed onto a solid basal medium consisting of M&S salts (9), 2% sucrose, 100 mg.l myi-uibisutik,m 1 mg/l thiamine-HCl, RT vitamine (7) and 0.8% agar (Technical No. 3, Oxoid), with -0.1 mg/indole-3-acetic acid (IAA) and four levels of 6-benzylaminopurine (6BA) : 01, 0.5, 1.0 and 5.0 mg/l (henceforth denominated as NB 01.01, NBB 01/10 and NB 01/50, respectively). The incubation ws carried out for 21 days under the same photoperiod conditions described for germination. Variables scored were the growth of callus (expressed as growth Index = weighted average of all the calli per treatment, calculated by assigning an arbitrary value, ranging from 0 to 4, to each callus growth estimated qualitiavely: 0 = no growth, 1 = little growth, 2 = middle growth, 3 = great growth, and 4 = enormous growth), and the organogenic response of the calli (expressed as percentage of calli giving shoot-buds).
Results:
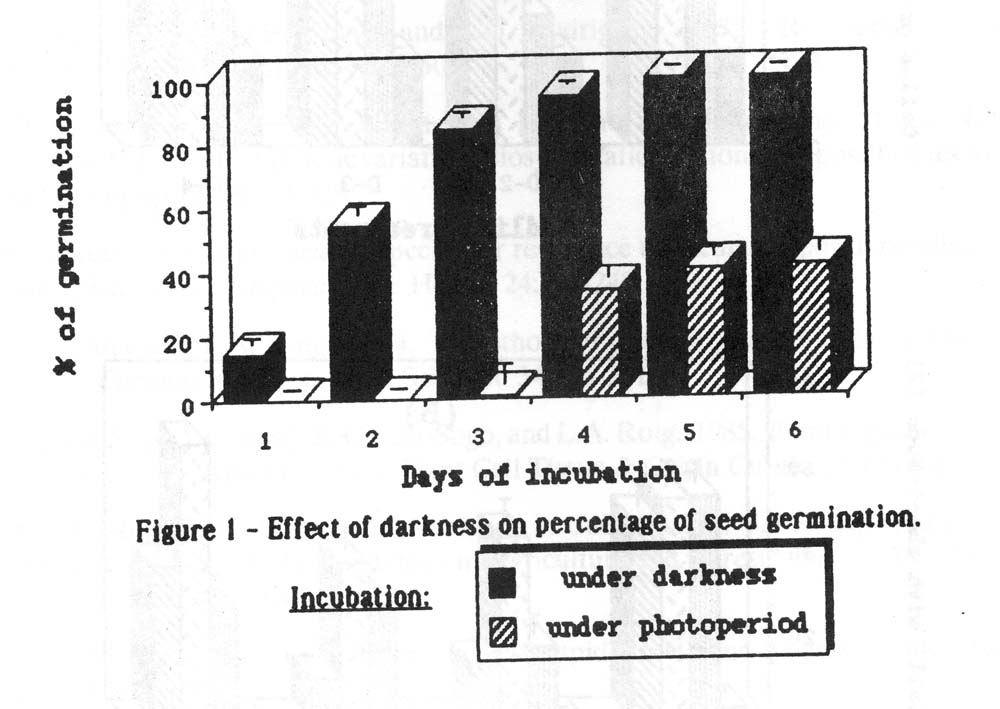
1. Seed germination. As can be seen in Figure 1, seeds incubated under photoperiod conditions present a germination rate much lower than those incubated under darkness and the time for germination is even longer. Thus, under photoperiod, germination does not occur until the 4th day of incubation and the maximum rate (41%) is reached after 6 days in a culture, whereas under darkness at 32 ˚ C a germination of 90% at the 3rd day and almost 100% after 4 days in culture can be obtained. Therefore, a simple change in the incubation conditions makes the quick attainment of high rates of germination feasible and also the assessment of the necessary amount of explant sources for further in vitro experiments.
Figure 1. Effect of darkness on percentage of seed germination.
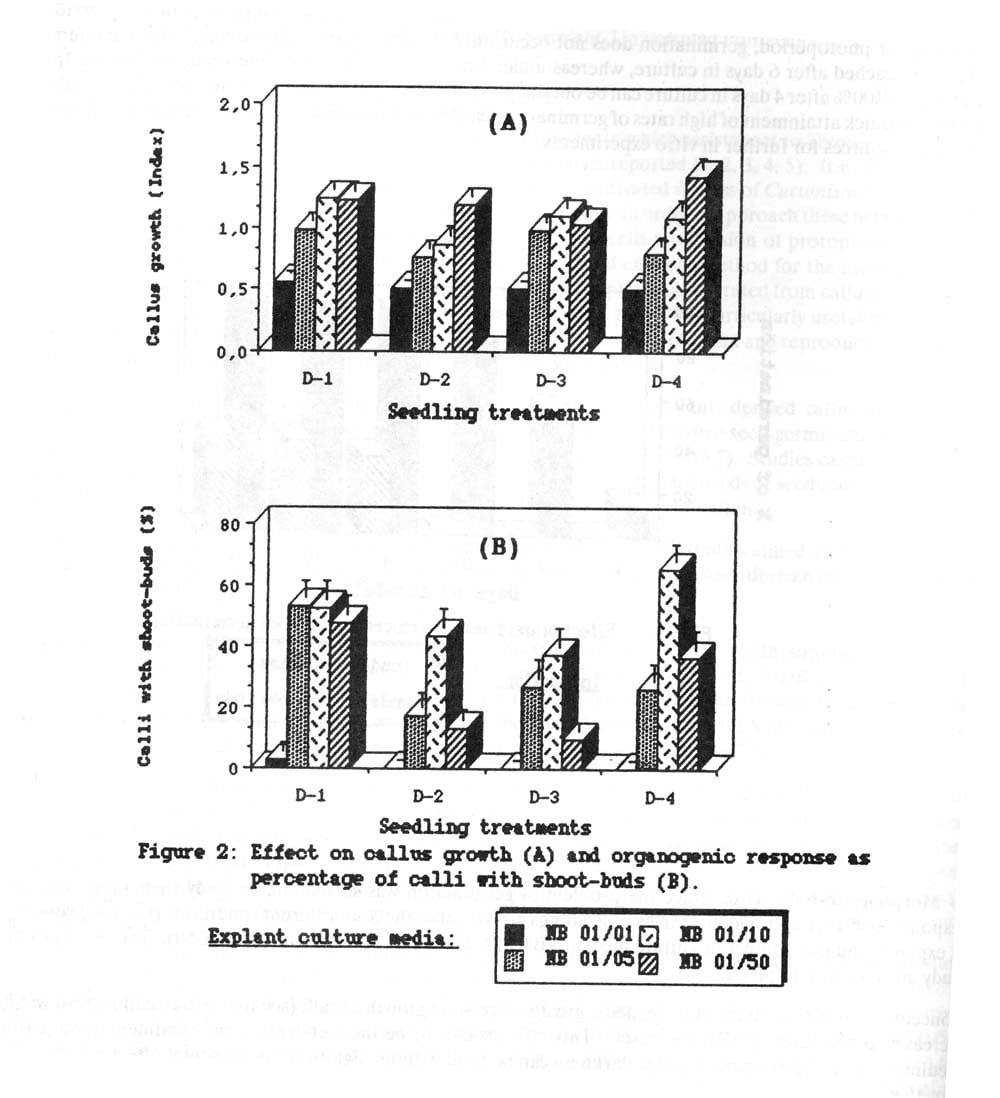
2. Morphogenesis in vitro. Once the problem of germinationw as solved and to study their morphogenetic response in vitro,. the cotyledon halves of seedlings grown under the four different conditions (D-1 – D4) were used as explants and put in the four culture media (NB 01/01, NB 01/05, NB 01/10 and NB 01.50). The results of this study are shown in Figure 2.
Concentration of 6BA in the culture media greatly affects the growth of calli (see figure 2A): callus fresh weight increases as the level of 6BA increases. This effect seems to be independent of any treatment given to the seedlings, so seed germination under darkness can be used without significant detrimental effect on the callus growth given that the appropriate culturemedium is selected.
The culture media also affects the organogenic response of the calli (Figure 2B), but in a different manner: with the lower level of 6BA (0.1 mg/l) there is no response at all, independently of seedling treatment, but the response at higher levels of 6BA does depend of the incubation conditions of the seedlings. Thus, when seedlings were germinated and grown under photoperiod, the 6BA concentration did not affect the regeneration rate (50-60% of the calli in all the cases), while when seeds were germinated under darkness, 6BA levels did not affect the results, being 1.0 mg/l (medium NB 01.10) the best of those studied, giving rise to frequencies of 40-7-% of calli forming shoot-buds, similar to those obtained under photoperiod. this means that by choosing this culture medium, one can use seeds germinated in darkness (at whatever time they were incubated) without decreasing the organogenic response of their explant-derived callus and ensuring the highest germination rate.
Figure 2. Effect on callus growth (A) and organogenic response as percentage of calli with shoot-buds (B).
In conclusion, both a method for overcoming the in vitro germination problems and the cultural conditions for the attainment of plant regeneration from cotyledon-derived calli of this wild species at high rates, have been achieved. Experiments aimed at obtaining biochemical markers through Agrobcterium-mediated transformation by applying these results, are in progress.
Acknowledgments: The authors express their appreciation to CICYT (Comision Interministerial de Ciencia y Tecnologia, Ministry of Education and Science, Spanish Government) for financial support (PRoject BIO89-0446). R. Santana is grrateful for her Grant from the Autonomous Government of the Valencian Community (Spain).
Literature Cited
- DePonti, O.M.B. 1978. Resistance in Cucumis sativus L. to Tetranychus urticae KOCH. I. Search for sources of resistance. Euphytica 27:167-176.
- Esteva, J. F. Nuez and M.L. Gomez-Guillamon. 1989. Resistance to yellowing disease in muskmelon. Cucurbit Genet. Coop. Rpt. 12:44-45.
- Knipping, P.A., C.G. Paterson, D.E. Knavel and L.G. Rodriguez. 1975. Resistance of cucurbits to twospotted spider mite. Environ. Entoml. 4:507-508.
- Kroon, G.H., J.B.M. Custers, Y.O. Kho, A.P.M. Den Nijs and H.Q. Varekamp. 1979. Interspecific hybridization in Cucumis L. I. Need for genetic variation. Biosystematic relations and possibilities to overcome crossability barriers. Euphytica 28:723-728.
- Lebeda, A. 1984. Screening of wild Cucumis species for resistance to cucumber powdery mildew (Ersiphe cichoracearum and Sphaerotheca fuliginea). Sci. Hortic. 24:241-249.
- Moreno, V., L. Zubeldia, an L.A. Roig. 1984. A method for obtaining callus cultures from mesophyll protoplasts of melon (Cucumis melo L.). Plant Sci. Lett. 34:195-201.
- Moreno, V., M. Garcia-Sogo, I. Granell, B. Garcia-Sogo, and L.A. Roig. 1985. Plant regeneration from calli of melon (Cucumis melo L. cv. Amarillo Oro). Plant Cell Tissue & Organ Culture, 5:139-146.
- Moreno, V. and L.A. Roig. 1990. Somaclonal Variation in Cucurbits. In: Y.P.S. Bajaj (ed.) Somaclonal Variation in Crop Improvement. I. Biotechnology in Agriculture and Forestry Series, Vol 11. Springer-Verlag Berlin Heidelberg, pp. 435-464.
- Murashige, T. and F. Skoog. 1962. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 15:473-497.