Cucurbit Genetics Cooperative Report 15:57-58 (article 21) 1992
W.A. Mackay, T. J. Ng and F.A. Hammerschlag
Department of Horticulture, University of Maryland, College Park, MD 20742, USDA, ARS, Plant Molecular Biology Laboratory, BARC-West, 10300 Baltimore Ave., Beltsville, MD 20442
Detached leaf inoculation assays by Kuti and Ng (2) of fifty melon cultigens revealed no source of resistance to Myrothecium roridum. However, in assays performed using both spore and roriden E inoculations there was a differential response between cultigens. Inheritance studies using diallel analysis indicated that tolerance or susceptibility was not simply inherited and there were no maternal effects (3). Greenhouse experiments were critically designated to further examine the mode of inheritance of tolerance to M. roridum in melon.
Parental, F1, F2, and backcross populations: Four melon populations consisting of the parents, F1, F2 , and backcrosses to both parents were derived from crosses of ‘Perlita’ x ‘Iroquois’, ‘Perlita’ x “Hales Best’, and from ‘Hales Best x ‘Iroquois’ and its reciprocal. The parents were crossed in the University of Maryland greenhouses in the winter of 1987-88 and the F2, BC1, and BC2 crosses made in the winter of 1988-89. Parental, F1 , F2, and backcross populations were seeded in square 9.2 cm pots in a commercial soilless mix (7.4 peat: 2.5 perlite: 1 vermiculite, v:v:v:) in the late winter and spring of 1990. Each population consisted of 10 plants of each parent and F1 , 200 plants of each F2, and 100 plants of each backcross.
Fungal culture: Myrothecium roridum (ATCC# 52485) was originally isolated from diseased melon fruit in Texas (1). Cultures were obtained from Dr. George Bean, Department of Botany University of Maryland, and maintained on PDA. Inoculum was obtained by placing mycelial plugs on fresh PDA and incubating under 16 hr photoperiods at 25˚ C. When sporulation occurred cultures were placed in the dark at 4 ˚ C until used. Spore suspensions were made by washing 2 or more plates with ddH20 and standardized to 106 spores per ml of distilled water.
Detached leaf inoculation: Leaf inoculations were performed when the plants had approximately ten true leaves. Expanded leaves approximately 9 cm in diameter were excised from the fourth or fifth node, washed with sterile distilled water three times, and placed in petri dishes lined with sterile moistened filter paper (2 ml sterile distilled water). Each leaf was inoculated with 25 μ l of spore suspension in an interveinal area and then covered with a moistened filter paper disc, 10 mm in diameter, for 24 hr. Inoculated leaves were incubated in a growth chamber at 25+ 1 ˚ C for seven days. Host reaction was determined by measuring necrotic lesion diameter and necrotic plus chlorotic lesion diameter.
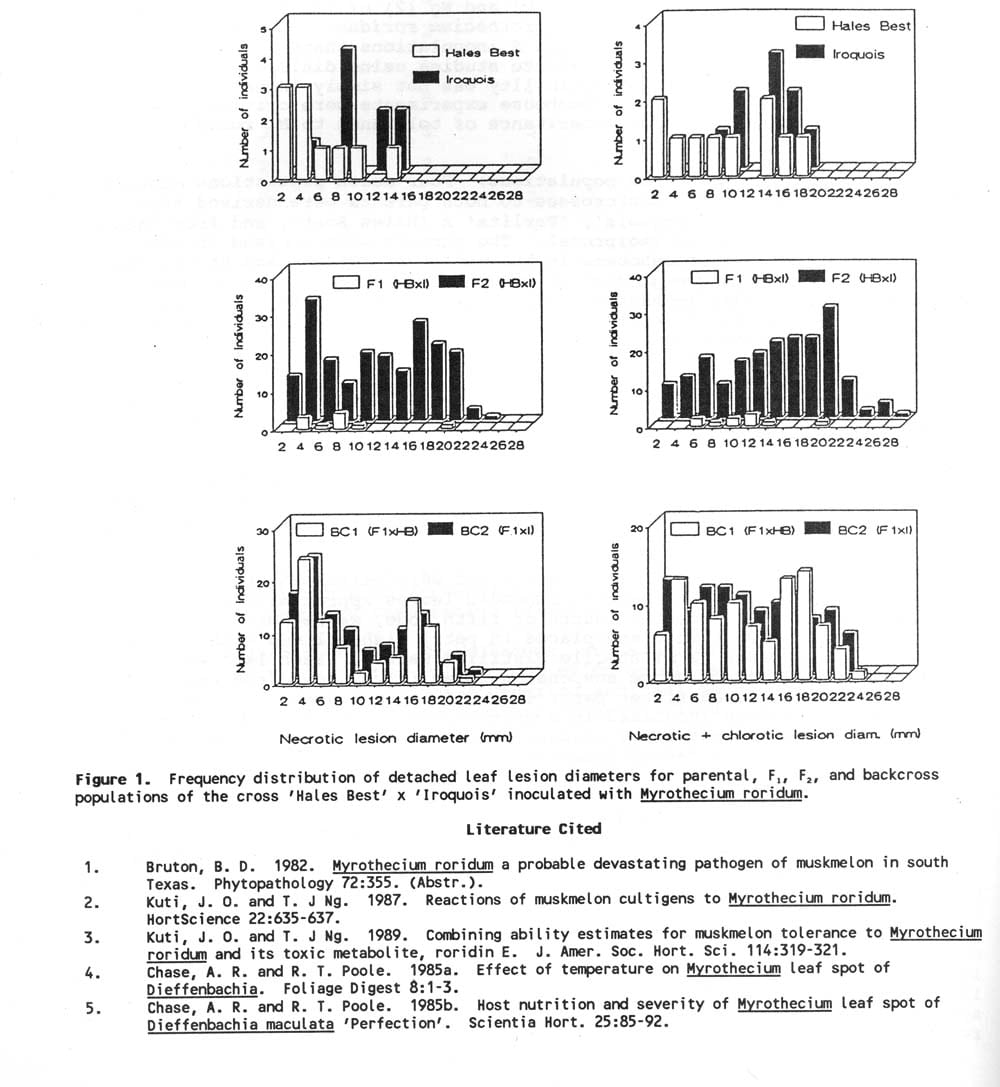
Results and Discussion: No conclusions can be made about the genetics of inheritance of melon resistance to M. roridum from these studies. Typical results are shown in Fig. 1. The backcross populations in each cross had similar distributions within each inoculation for both necrotic and necrotic plus chlorotic lesion measurements. In general there was no clear difference between the parent populations. In one case ‘Hales Best’ and ‘Iroquois’ had opposite responses for the two inoculation dates. The F1 and F2 populations generally had the same distribution of individuals, although the small numbers of F1 individuals made comparison difficult. The amount of time between inoculation dates may be important in obtaining repeatability of results. The inoculation dates may be important in obtaining repeatability of results. The inoculation two days apart (5/23 and 5/25) had very similar population responses while other inoculations with greater time intervals had less similar responses. However, there was insufficient data to test this hypothesis.
Studies with Myrothecium roridum have shown that host reaction can vary with changes in the host plant nutrition and growing conditions (4, 5). Kuti (personal communication) indicated that the pathogenicity of the melon isolate of M roridum is affected by host factors such as age, position of leaf on the plant, and general health of the plant. Host reaction to Myrothecium roridum may have been influenced by the presence of a virulent strain of powdery mildew in the greenhouses; a number of leaves were discarded due to obvious infestation of the leaves with powdery mildew shortly after inoculation with M. roridum. Powdery mildew appeared to suppress the progress of M. roridum on those leaves that were heavily infested. However, since these leaves were often discarded before the end of the incubation period no data was collected.
Figure 1. Frequency distribution of detached leaf lesion diameters for parental, F1 , F2 , and backcross populations of the cross ‘Hales Best’ x ‘Iroquois’ inoculated with Myrothecium roridum.
Literature Cited
- Bruton, B.D. 1982. Myrothecium roridum a probably devastating pathogen of muskmelon in south Texas. Phytopathology 72:355. (Abstr.).
- Kuti, J.O. and T.J. Ng. 1989. Reactions of muskmelon cultigens to Myrothecium roridum. HortScience 22:635-637.
- Kuti, J.O. and T.J. Ng. 1989. Combining ability estimates for muskmelon tolerance to Myrothecium roridum and its toxic metabolite, roridin E. J. Amer. Soc. Hort. Sci. 114:319-321.
- Chase, A.R. and R.T. Poole. 1985a. Effect of temperature on Myrothecium leaf spot of Dieffenbachia. Foliage Digest 8:1-3.
- Chase, A.R. and R.T. Poole. 1985b. Host nutrition and severity of Myrothecium leaf spot of Dieffenbachia maculata ‘Perfection’. Scientia Hort. 25:85-92.