Cucurbit Genetics Cooperative Report 18:13-15 (article 6) 1995
K. Niemirowicz-Szczytt, M. Rucinska, A. Korzeniewska and S. Malepszy
Department of Genetics and Hort. Plant Breeding, Warsaw Agricultural University, SGGW, 02-766 Warsaw, Poland
As with previously described mutants (2-5), the two mutants reported here belong to our collection developed by Kubicki. Mutants were obtained by ethyleneimine seed treatment of the inbred Borszczagowski (B) line.
The female sterile mutant could be distinguished in the seedling stage (Figure 1) by a long hypocotyl and two true leaves which arose from the first internode. Mature mutant plants were less robust, with shorter main stems but longer internodes and petioles than controls (Table1). In general, plants produced fewer lateral branches (Figure 2); their leaves were smaller, generally because they were more narrow.
The mutant was monoecious with normally developed male and female flowers. Pollen stainability was up to 98% and viable seeds could be obtained after backcrosses to inbred line (B) or crosses with heterozygous plants. In contrast, female flowers self-pollinated or cross-pollinated with genotypically different lines occasionally developed deformed fruits but never set seeds (Figure 3). Cytoembryological analysis of young ovules indicated early degeneration of the embryo sac. Genetic analysis (Table 2) indicated that a single recessive gene (fs – female sterile) regulates the phenotype described above.
A long hypocotyl mutation was previously described by Robinson and Shail (1), as a result of neutron radiation of “Lemon” seed. Our independent mutation was a result of chemical seed treatment of the Borszczagowski line. Phenotypicaly, our long hypocotyl mutation was similar to that described earlier (1). Mutation was evident in the seedling stage due to the long hypocotyl. The mains tem and leaf petioles were longer than that of control and leaf blades were larger (Table 3). Other traits were not changed in comparison to inbred “B” line. Genetic analysis indicates (Table 4) that a single recessive gene also regulates this phenotype; we have designated this gene (lh2).
Plants of similar phenotype were obtained as a result of somaclonal variation. Crosses between these two long hypocotyl plants gave long hypocotyl progeny. This suggests that the locus was easy to mutate and that our chemically induced mutation is probably allelic to that obtained in course of tissue culture.
Figure 1. Long hypocotyl (right) and female sterile (middle) mutant seedlings, compared to the wild type seedling (left).
Figure 2. Female sterile cucumber plant with one deformed fruit.
Figure 3. A deformed fruit with no evidence of seeds from a female sterile mutant plant.
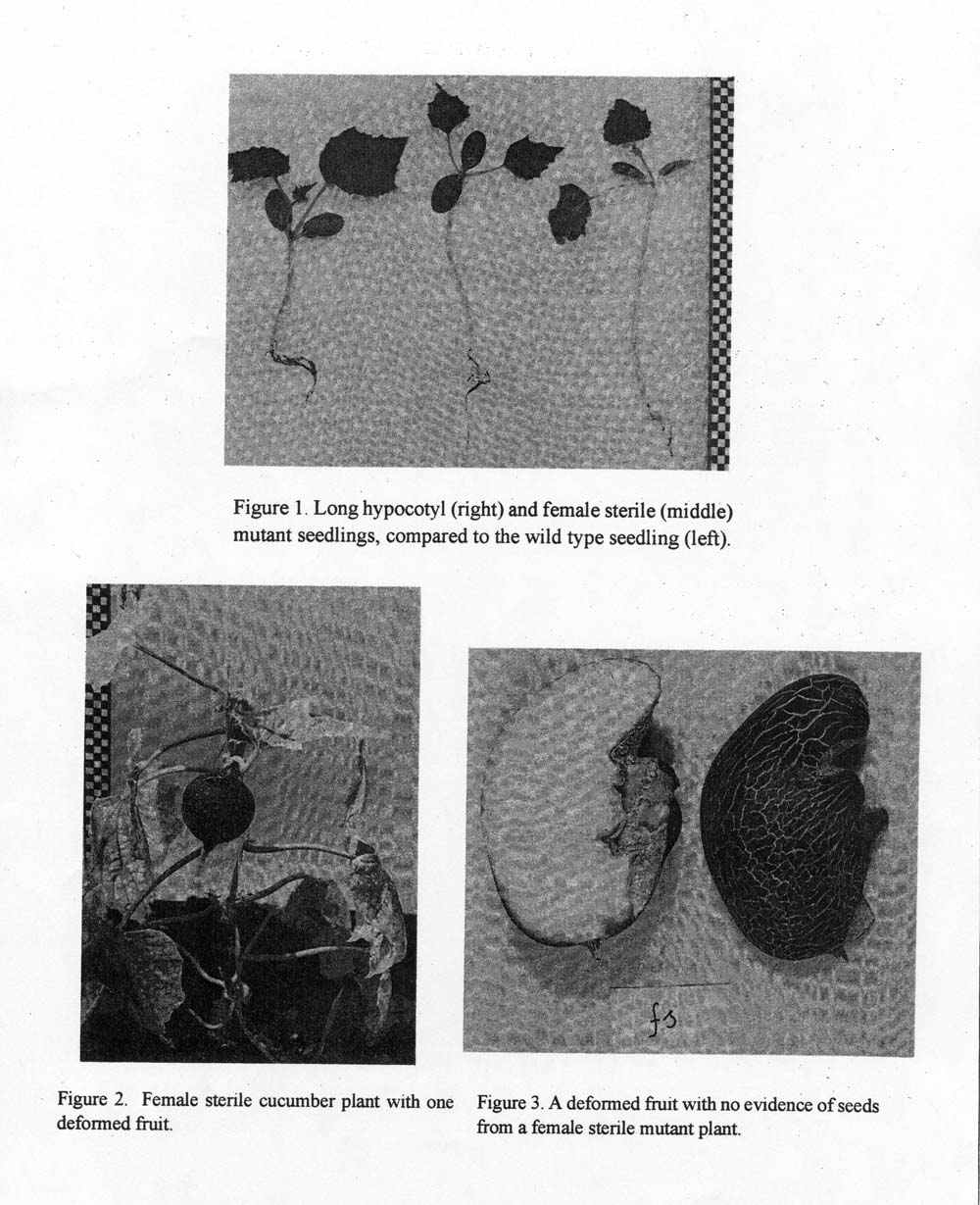
Table 1. Measurements (cm) for five characters of twenty mutant (fs) and twenty normal (B) cucumber plants.
Plant type |
Hypocotyl length |
Plant height |
5th Leaf |
||
Lamina |
Petiole Length |
||||
Length |
Max. Width |
||||
| Normal (B) | 13.3 + 1.6 | 225.8 + 21.2 | 15.5 + 3.8 | 20.8 + 0.8 | 20.0 +1.1 |
| Mutant (fs) | 18.1 + 2.5 | 151.9 + 13.1 | 13.2 + 3.2 | 15.1 + 3.2 | 29.2 + 6.7 |
Table 2. Inheritance of female sterility (fs)
Generation |
Normal |
No. observed Mutated |
Normal |
No. expected Mutated |
Ratio tested |
X² |
P |
| P1 (normal) | 22 | 0 | 22 | 0 | 1:1 | – | – |
| P2 (mutated) | 0 | 40 | 0 | 40 | 0:1 | – | – |
| F1 | 20 | 0 | 20 | 0 | 1:0 | – | – |
| F2 | 140 | 42 | 136.5 | 45.5 | 3:1 | 0.42 | 0.05 |
| F1 x P1 | 78 | 0 | 78 | 0 | 1:0 | – | – |
| F1 x P2 | 54 | 60 | 67 | 57 | 1:1 | 0.21 | 0.05 |
Table 3. Measurements (cm) for five characters of twenty mutant (lh2) and twenty normal (B) cucumber plants.
Plant type |
Hypocotyl length |
Plant height |
5th Leaf |
||
Lamina |
Petiole Length |
||||
Length |
Max. width |
||||
| Normal (B) | 13.3 + 1.9 | 225.8 + 21.2 | 15.5 + 3.8 | 20.0 + 0.8 | 20.0 + 1.1 |
| Mutant (lh2) | 19.3 + 1.9 | 375.6 + 26.9 | 19.9 + 1.0 | 25.0 + 1.4 | 40.2 + 5.8 |
Table 4. Inheritance of long hypocotyl (lh2)
Generation |
Normal |
Mutated |
Normal |
Mutated |
tested |
X² |
P |
| P1 (normal) | 22 | 0 | 22 | 0 | 1:0 | — | — |
| P2 (mutant) | 0 | 21 | 0 | 21 | 0:1 | — | — |
| F1 | 25 | 0 | 25 | 0 | 1:0 | — | — |
| F2 | 124 | 46 | 127.5 | 42.5 | 3:1 | 1.44 | 0.05 |
| F1 x P1 | 79 | 0 | 79 | 0 | 1:0 | — | — |
| F1 x P2 | 34 | 37 | 35.5 | 35.5 | 1:1 | 0.13 | 0.05 |
Literature Cited:
- Robinson, R.W., and J.W. Shail. 1981. A cucumber mutant with increased hypocotyl and internode length. Cucurbit Genet. Coop. Rpt. 4:19-20.
- Rucinska, M.K., Niemirowicz-Szczytt and K. Korzeniewska. 1991. A cucumber (Cucumis sativus L.) mutant with yellow stem and leaf petioles.
- Rucinska, M., K. Niemorowicz-Szczytt and A. Korzeniewsia. 1992. Cucumber (Cucumis sativus L.) induced mutations. II. A second short petiole mutant. Cucurbit Genet. Coop. Rpt. 15:33-34.
- Rucinska, M., K. Niemirowicz-Szczytt and A. Korzeniewska. 1992. Cucumber (Cucumis sativus L.) induced mutations. III and IV. Divided and ginko leaves. Fifth Eucarpia Cucurbitaceae symposium, Poland, July 27-31, 1992. pp.66-69.
- Rucinska, M., E. Berger, K. Niemirowicz-Szczytt and A. Korzeniewska. 1993. Cucumber (Cucumis sativus L.) induced mutations: A Phaseolus leaf mutant. Cucurbit Genet. Coop. Rpt. 16:1415.