Cucurbit Genetics Cooperative Report 19:1-3 (article 1) 1996
K. Niemirowicz-Szczytt, M. Rucinska and A. Korzeniewsia
Department of Plant Genetics, Breeding and Biotechnology, Warsaw Agricultural University, SGW, 02-787 Warsaw, Poland
A mutant designated as super compact (scp) belongs to the series of mutants (1,3-6) which are currently being maintained in a collection originally developed by B, Kubicki. As with all mutants in this collection, the super compact was obtained by ethyleneimine seed treatment of the inbred line ‘Borszczagowski’ (B). The morphological description of this mutant provided herein was based on 20 plants. The genetic analysis defining its inheritance was based on several hundred individuals.
The super compact mutant can be included in the broad group of growth-type mutants which have drastically reduced main stem length. This mutant can be distinguished in the seedling stage (Fig. 1) by a drastic decrease in shoot length when compared to the standard wild-type. However, it possesses a hypocotyl the standard size as that of the wild type.
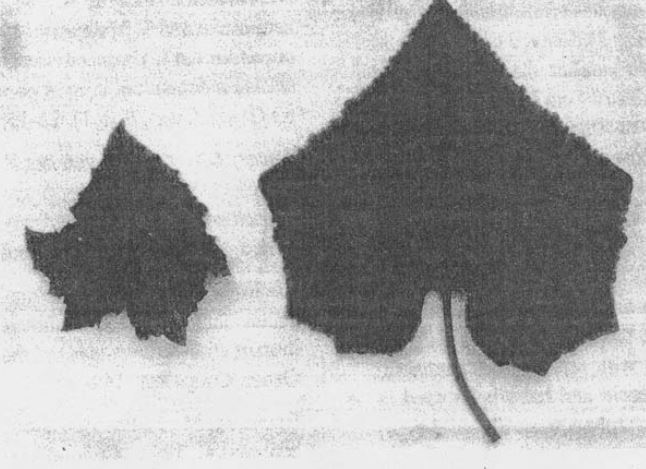
Fully developed plants of the super compact mutant are small [4.7 + 0.6 cm high (Fig. 2) when compared to the B line (266.0 + 6.2 cm). While the length of the internodes of this mutant ranges between 0.2 to 0.5 cm, that of the B line is between 6.1 to 7.4 cm. The mutant plant does not develop lateral branches while the B line possesses lateral branches (24 + 3).Leaves of this mutant are dark green and a little smaller than the standard wild-type (mutant leaves 13 to 14 cm long, and 15 to 17 cm wide with leaf petiole 9 to 10 cm long, and respective dimensions of standard wild-types are 16 to 17, 20 to 22, 16 to 18 cm). The smaller leaves with shorter petioles typical of the mutant are thicker and slightly wrinkled when compared to the wild-type (Fig. 3). Flower petals of this mutant are wrinkled, and gynoecious flowers develop rarely. The pistils are deformed and never develop into fruit. Staminate flowers of this mutant often contained deformed stamens with fertile (90% stainable) pollen grains which are viable and have been used in backcrosses to heterozygous (phenotypically wild-type) individuals.
The F1 data presented in Table 1 confirm the recessive character of spc. However, deviation from the expected ratios was observed in F2 and BCP2 families. Deviations from expected ratios may be due to a lower germination ability of mutant seeds and lower seedling resistances to common cucumber pathogens.
Table 1. Inheritance of super compact (scp) gene in cucumber.
No. Observed |
No. expected |
Ratio tested |
X2 |
p |
|||
Generation |
Wild type |
Mutant |
Wild Type |
Mutant |
|||
| P1 (Normal) | 39 | 0 | 39 | 0 | 1:1 | — | |
| P2 (Mutant) | 0 | 50 | 0 | 50 | 0:1 | — | |
| F1 | 53 | 0 | 53 | 0 | 1:0 | — | |
| F2 | 212 | 40 | 189 | 63 | 3:1 | 11.19 | 0.05 |
| BC P1 | 181 | 0 | 181 | 0 | 1:0 | — | |
| BC P2 | 91 | 87 | 79 | 79 | 1:1 | 1.00 | 0.05 |
The mutant described herein may be identical to the rosette (ro) mutant described by de Ruiter et al. (2). The mutant description given by these researchers is very general, and thus it is difficult to ascertain whether it is genetically identical to the sep mutant. The origin of rosette is different, since it was identified as a segregant in megurk cross progeny. Traits useful in distinguishing the rosette and super compact mutants include rosette’s ability to set fruits after self-pollination indicating that female flowers of rosette are anatomically normal. The availability of rosette seeds would allow for allelism tests to be made. Such seeds may be available since the rosette mutant was used relatively recently by Zijlstra in 1987 (7) for linkage studies in Cucumis sativus.
Figure 1. The super compact mutant (right) compared to wild type seedling (left).
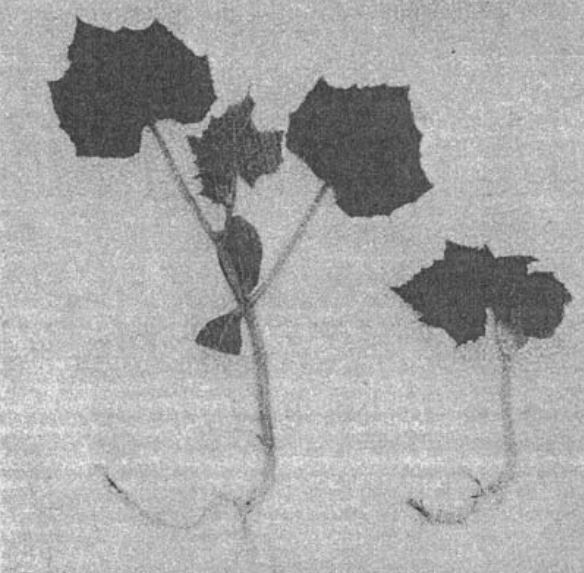
Figure 2. The mature super compact mutant plant.

Figure 3. A leave-size comparison of the super compact mutant (left) and standard wild type (right).
Literature Cited
- Niemirowicz-Szczytt, L., M. Rucinska, A. Korzeniewska and S, Malepszy. 1995. Cucumber (Cucumis sativus L.) induced mutations: A female sterile and an independent long hypocotyl mutant. Cucurbit Genet. Coop. Rpt. 18:13-15.
- Ruiter, A.C. de, B.J. van der Knapp and R.W. Robinson. 1980. Rosette, a spontaneous cucumber mutant arising from cucumber-muskmelon mentor pollen. Cucurbit Genet. Coop. Rpt. 3:4.
- Rucinska, M., K. Niemirowicz-Szczytt and A. Korzeniewska. 1991. A cucumber (Cucumis sativus L.) mutant with yellow stem and leaf petioles. Cucurbit Genet. Coop. Rpt. 14:8-9.
- Rucinska, M., K. Niemirowicz-Szczytt and A. Korzeniewska. 1992. Cucumber (Cucumis sativus L.) induced mutations. II. A second short petiole mutant. Cucurbit Genet. Coop. Rpt. 15:33-34.
- Rucinska, M., K. Niemirowicz-Szczytt and A. Korzeniewska. 1992. Cucumber induced mutations. III and IV. Divided and ginko leaves. Fifth Eucarpia Cucurbitaceae Symposium, Poland. July27-31, 1992. pp. 66-69.
- Rucinska, M., K. Niemirowicz-Szczytt and A. Korzeniewska. 1993. Cucumber (Cucumis sativus L.) induced mutations: A Phaseolus leaf mutant. Cucurbit Genet. Coop. Rpt. 16:14-15.
- Zijlstra, Z. 1987. Further linkage studies in Cucumis sativus. Cucurbit Genet. Coop. Rpt. 10:39.