Cucurbit Genetics Cooperative Report 19:38-41 (article 15) 1996
G. Sapountzakus and A.S. Tsaftaris
Dept. Genetics and Plant Breeding, Aristotelian University of Thessaloniki, Greece
Introduction. Cucumber can be infected with Agrobacterium and through this infection can be genetically transformed. Transgenic cucumber plants of the cultivar Straight 8 expressing the selection neomycin phosphotransferase II (npt II) gene were regenerated through inoculation with a strain of Agrobacterium rhizogenes (9). The transfer and expression of Cucumber Mosaic Virus coat protein gene in the genome of cultivar Poinsett 76 was obtained using an Agrobacterium tumefaciens mediated transformation and expression of neomycin phosphotransferase II were studied in two inbred lines of pickling cucumber (8).
The present report describes the use of a disarmed strain of Agrobacterium tumefaciens to mediate the transfer and expression of a reporter gene coding for luciferase in the genome of the cucumber hybrid Bambina. It also describes the use of regeneration via shoot organogenesis to obtain transformed plans that express the luciferase gene.
Materials and Methods. A modified Agrobacterium tumefaciens strain LBA 4404 which contains the disarmed Ti plasmid pAL 4404 (4) was used. This strain contained an additional plasmid (pAQ2 ) which was constructed by modification of the binary Ti vector BIN 19 (1) through addition of the plasmid T-DNA with the firefly luciferase reporter gene supplemented with CaMV promoter (6). The T-DNA region also contained the kanamycin-resistance selectable marker gene (nptII) driven by the nopaline synthase (nos) promoter (2). A bacterial kanamycin-resistance gene (npt III) driven by a bacterial promoter was cloned outside the borders of the T-DNA region.
Seeds of the Bambina (De Ruiter Seeds) cucumber hybrid were soaked in a Triton x 100 (Merck) 0.01% v/v/solution for 20 min. They were subsequently surface sterilized in a 20% v/v solution of commercial bleach (5% sodium hypochlorite) for 15 min. The seeds were then rinsed three times with sterile distilled water and placed under aseptic conditions in petri dishes containing 0.8% agar-agar (Sigma) in darkness at 25 C for five to seven days to germinate. Unless otherwise stated all media were solidified with 0.8% agar. The pH of the media was adjusted to 5.7 before autoclaving at 121 C for 20 min.
Five-to seven-day-old in vitro grown seedlings were used as donors of cotyledon explants. Cotyledons were divided in two parts: proximal and distal to the embryonic axis ones. The distal parts were discarded and the proximal were divided in two parts with a cut across the central vein. These explants were submerged overnight in an MS (Murashige and Skoog) (5) liquid medium supplemented with 4 mg/l 6-benzylaminopurine (BA) in which bacterial cells of the previously referred to strain were diluted. bacterial cells for infections were produced after an overnight culture in a liquid LB medium containing 100 mg/l kanamycin at 28 C and vigorous shaking. An aliquot of 1.5 ml of this culture was then centrifuged for 7 min. at 10,000 rpm and the bacterial pellet was diluted in a 50 mm diameter petri dish containing 5 ml of the previous referred to liquid medium supplemented with BA. Eight cotyledon segments were then added to this petri dish.
Twenty-four hours later the cotyledon explants were rinsed five times with distilled sterile water, blot dried on sterile filter papers and cultured upside down in sterile petri dishes on solid MS medium supplemented with 4 mg/l BA. This medium was found to be optimum for the regeneration of parthenocarpic cucumber hybrids such as ‘Bambina’ and ‘Brunex’ (7). After two days the explants were transferred on a MS medium supplemented with 4 mg/l BA, 50 MG/l kanamycin and 250 mg/l BA + 100 mg/l kanamycin + 500 mg/l cefotaxime. Every ten days the explants were re-transferred to petri dishes with the previously referred to medium. Kanamycin was used to select the transformed plant cells and cefotaxime to control the growth of bacteria which remained on the cotyledon surface after the co-cultivation period. The shoots which were produced (Fig. 1) were transferred to magenta vessels (SigmaP on MS media supplemented with 100mg/l kanamycin and 500 mg/l cefotaxime, where they were elongated and rooted (Fig. 2).
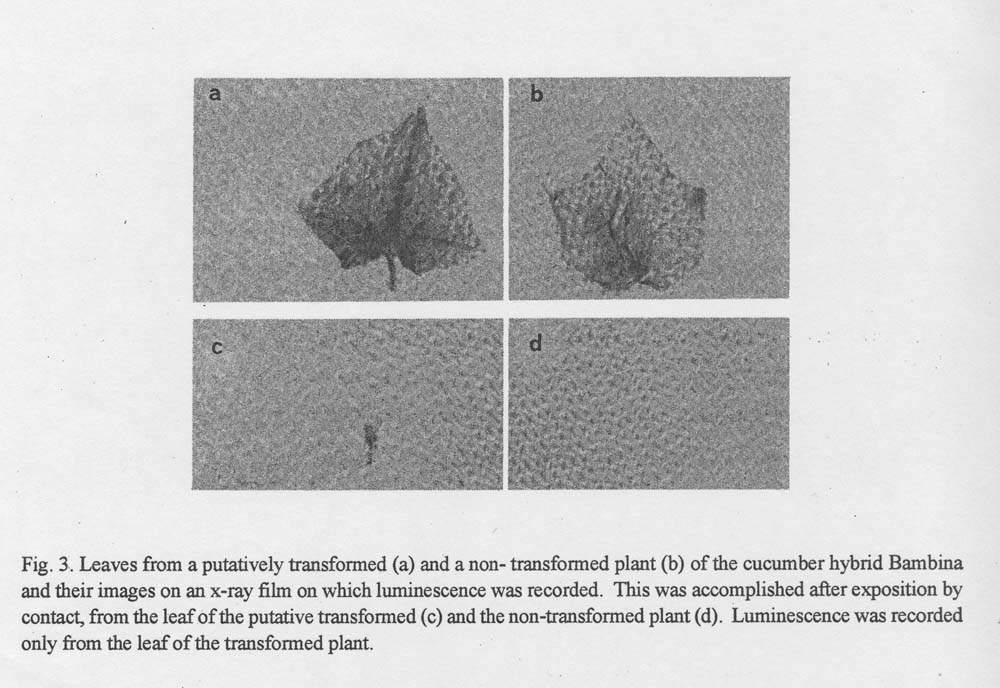
Leaves from the putative transformed plants were soaked for 2.5 h in 0.1 mM luciferin solution containing 100 mM sodium citrate (pH 5) and 20% v/v dimethyl sulfoxide and then exposed by contact to x-ray film (Kodak OG) (6) for 24h. The leaf luminescence was recorded on the x-ray film (Fig. 3) to document the transfer and expression of the CaMV promoter and the firefly luciferase gene in the genome of the cucumber hybrid Bambina.
Results and Discussion. The transfer and expression of an easily manipulated and early detectable reporter gene was performed to standardize a protocol for cucumber genetic transformation. This procedure may be used for transferring genes which code for economically important traits (e.g., resistance to viruses and pests).
Fig. 1. Putative transformed shoot regenerated from cotyledon segment of the cucumber hybrid Bambina three weeks after co-cultivation with recombinant bacteria.
Fig. 2. Putative transformed plantlet of the cucumber hybrid Bambina three months after co-cultivation with recombinant bacteria.
Fig. 3. Leaves from a putatively transformed (a) and a non-transformed plant (b) of the cucumber hybrid Bambina and their images on an x-ray film on which luminescence was recorded. This was accomplished after exposition by contact, from the leaf of the putative transformed (c) and the non-transformed plant (d). Luminescence was recorded only from the leaf of the transformed plant.
Literature Cited
- Bevan, M. 1984. Binary Agrobacterium vectors for plant transformation. Nucleic Acid Res. 12:8711-8721.
- Bevan, M.W., R.B. Flavell and MD. Chilton. 1983. A chimeric antibiotic resistance gene as a selectable marker for plant cell transformation. Nature 304:184-187.
- Chee, P.P. and J.L. Slightom. 1991. Transfer and expression of Cucumber Mosaic Virus coat protein gene in the genome of Cucumis sativus. J. Amer. Soc. Hort. Sci. 116:1098-1102.
- Hoekeme, A. P.R. Hirsch, P.J.J. Hoykaas and R.A. Schilperoort. 1983. A binary plant vector strategy based on separation of vir- and T- region of the Agrobacterium tumefaciens Ti plasmid. Nature 303-179-180.
- Murashige, T. and F. Skoog. 1962. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 15:473-479.
- Ow, D.W., K.V. Wood, M. DeLuca, J.R. De Wet, D.R. Helinski and S.H. Howell. 1986. Transient and stable expression of the firefly luciferase gene in plant cells and transgenic plants. Science 234:856-859.
- Sapountzakis, G., E. Nianou-Obeidat and A.S. Tsaftaris. In vitro shoot regeneration from cotyledon explants of the parthenocarpic cucumber (Cucumis sativus L.) hybrids ‘Brunex’ and ‘Bambina’. (in preparation).
- Saramento, G.G., K. Alpert, F.A. Tang and Z.K. Punja. 1992. Factors influencing Agrobacterium tumefaciens mediated transformation and expression of kanamycin resistance in pickling cucumber. Plant Cell, Tissue and Organ Cult. 31:185-193.
- Trulson, A.J., R.B. Simpson and E.A. Shahin. 1986. Transformation of cucumber (Cucumis savitus L.) plants with Agrobacterium rhizogenes. Theor. Appl. Genet. 73:11-15.