Cucurbit Genetics Cooperative Report 21:14-15 (article 5) 1998
Yu Shuancang
Beijing Vegetable Research Center. Beijing, 100081, P.R. China
Cui Hongwen
Department of Horticulture. Northwestern Agricultural University, Yangling, Shaanxi, 712100, P.R. China
Introduction. As apparently universal response to stress by plants is the production of ethylene. In addition to “normal” production of ethylene, as in fruit ripening, plants produce ethylene when they are stressed by low temperature (1). Stress ethylene is generally produced before visible symptoms appear, and appears to be a transitory phenomenon (1, 2). When stress is removed, ethylene production normally reverts back to normal within 24 hr. Many studies have shown that the extent to which ethylene is released after chilling could provide a selection tool which would allow the ranking of different genotypes for tolerance to low temperature (1, 2, 3), although this application may be limited to some degree. The serviceability of this approach was investigated in the current study.
Methods. Three cucumber (Cucumis sativus L.) accessions were used in the trials: ‘Pingli’ (chilling tolerant), ‘Xinong-145’ (moderately tolerant) and ‘Jinyan-4’ (chilling susceptible). Eight-day-old cucumber seedlings were acclimated under 12 hr day length with day and night temperatures of 15 C and 10 C, respectively, for 2 days. Seedlings were then chilled at 2.5 C for 24, 48 or 72 hr., and transferred to a 12 hr day length “resuming period” (day/night temperatures of 25 C and 18 C, respectively) prior to ethylene measurements. Four uniform seedlings per sample were sealed in 20 ml glass jars for 2 hr before ethylene measurement. Gas samples of 1 ml were taken from the jars and measured by gas chromatography. All tests were replicated twice.
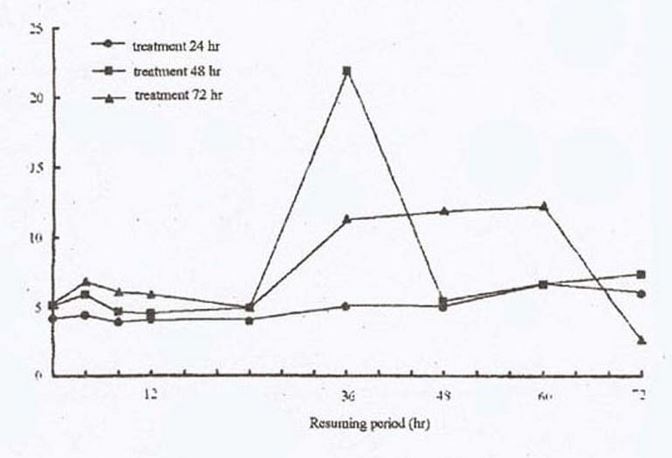
Results. During the resuming period, ‘Xinong-145’did not produce abnormal amounts of ethylene after chilling (2.5 C) for 24 hr. With low temperature treatment for 48 hr. or 72 hr, during the resuming period there appeared two peaks of ethylene production within 72 hr. The two peaks appeared after resuming for 4 hr. and 36-48 hr respectively. The first ethylene peak for he 72 hr treatment was higher than for the 48 hr treatment. However, the 2nd ethylene peak for the 72 hr treatment was lower than the 48 hr treatment, but its duration was longer. Therefore, the optimum stress condition for the detection of stress ethylene production appears to be 2.5 C for 72 hr (Fig. 1).
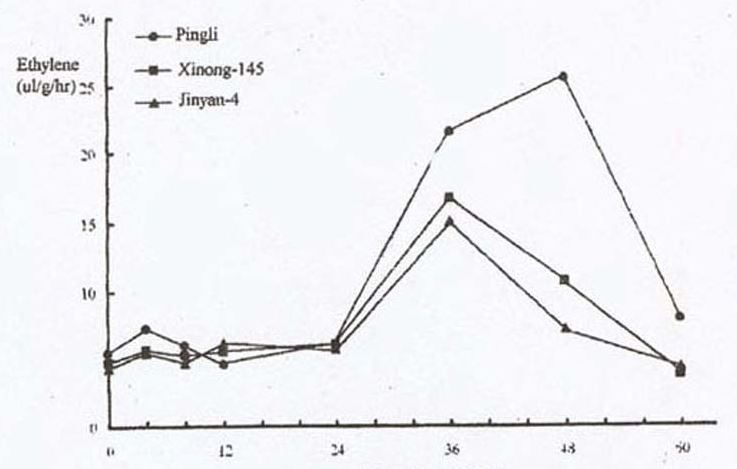
Similar results were obtained when seedlings of the three cucumber accessions were chilled for 72 hr (Fig. 2). There were bursts of ethylene production during the resuming period, and peaks measured during the resuming periods of 4 hr and 36-48 hr corresponded well with the tolerance ability to low temperature in cucumber. The most tolerant cultivar, ‘Pingli’, had the highest ethylene peak value.
Based upon these experiments, when ethylene is to be used in the identification of low temperature tolerant ability in cucumber, the standardized detection program should be as follows: treat 8-day-old seedlings at 2.5 C for 72 hr, then detect ethylene production after resuming for 4 hr or 36-48 hr.
Discussion. Ethylene production was induced by low temperature, and may be an adaptation of the plant to chilling temperatures. During the resuming period after chilling, there were two peaks of ethylene production within 72 hr, and the second peak was accompanied by the physical breakdown of the seedling. It has been suggested that the amount of ethylene produced after chilling could serve as a selection indicator for tolerance to low temperature. If so, this technique could have many advantages, such as ease of measurement, high sensitivity, lack of interference from other metabolites, and non-destructiveness to the seedlings. The further development of this technique may result in increased efficiency for breeding for low temperature tolerance in cucumber.
Literature Cited
- Chen, Yi-Zhu and Brian D. Patterson. 1985. Ethylene and 1-aminocyclopropane-1carboxylic acid as indicators of chilling sensitivity in various plant species. Aust. J. Plant Physiol. 12:377-385.
- Wang, C.Y. and D.O. Adams. 1982. Chilling-induced ethylene production in cucumbers. Plant Physiol. 69:424-427.
- Liu, Jianhui, Hongwen Cui, et al. Effect of chilling on ethylene release and electrolyte leakage of cucumber seedlings among different chilling sensitive cultivars. Acta Univ. Agric. Boreall-occidentalis 22(4):61-64.