Cucurbit Genetics Cooperative Report 22:1-2 (article 1) 1999
H. Mizusawa and S. Matsuura
Tohoku Seed Co., 1625 Himuro, Nishihara, Utsunomiya 321-3232, Japan
S. Kamachi and S. Sakai
Institute of Biological Sciences, University of Tsukuba, Ibaraki, 305-8527, Japan
In Japan, monoecious cucumber is very popular, and there are commercial varieties that possess various degrees of monoecious sex expression (i.e. ratio of pistillate to staminate flowers) is a quantitative trait and cross breeding for line development of stable monoecious sex types is difficult. Therefore, a selectable marker is needed to enhance breeding of monoecious sex phenotypes. Recently, it was suggested that ACC synthase (CS-ACSIG) is closely linked to or is the F-locus (gynoecious gene) (1), and that ACC synthase (CS-ACS2) is also associated with sex phenotypes in cucumber (2). In this report, we examined the relationship betweenCS-ACS2 and the monoecious sex phenotype using an F2 population derived from a cross between monoecious cucumber lines high and low expression of staminate flowers.
Materials and Methods.
An F2 population (130 individuals) was derived from a cross between ‘Kyuuraku No.2’ and ‘Morioka No. 1’. The parents and F1 as well as F2 progeny were also used for experimentation. All plant materials were planted in a greenhouse in Utsunomiya, Japan, in the summer of 1995. Details of cultivation are given described in the Proceedings of Cucurbitaceae ’98 (3). The number of female flowers appearing from the first to 17th node of the main stem were recorded for each plant.
Total DNA was isolated from the shoot apex of the lateral stem by CTAB method (4). total DNA of both parents were independently digested with nine restriction enzymes (Bam H1, Dra 1, Eco R1, Eco RV, Hind III, Sac I, Sal I and Xho 1). Thereafter, 3 μg DNA from each individual was electrophoresed through 0.8% agarose gel, and then transferred nylon membrane (Amersham). The cDNA clone of CS-ACS2 was used as a probe for Southern blot analysis after being labeled with an ECL gene detection kit (Amersham). Membranes were hybridized overnight at 42 C in a hybridization buffer that was in the ECL kit. They were washed twice in a primary wash buffer that contained 0.5 x SSC, 0.4% SDS nd 6M urea at 42 C for 20 min, and then twice in a secondary wash buffer that contained 1.0 c SSC at room temperature for 10 min. The membranes were exposed to Fuji medical X-ray film for 1h after being soaked with a detection solution. Mean separations of the number of female flowers for each CS-ACS2 genotype in F2 population were performed using LSD tests.
Results and Discussion.
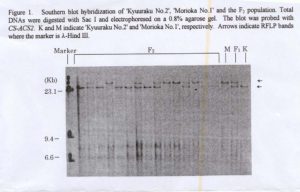
An RFLP was detected between ‘Kyuuraku No. 2’ and ‘Morioka No. 1’ when their total DNAs were digested with Sac I enzyme as shown in Figure 1. The segregation of CS-ACS2 in F2 population (total DNAs were isolated from only 79 individuals) fitted a 1:2:1 ratio as shown in the Table 1. This results suggested that there is at least one additional copy of CS-ACS2 in cucumber. The number of female flowers of ‘Kyuuraku No. 2’, ‘Morioka No. 1’, the F1 hybrid and in each CS-ACS2 F2 genotype of are shown in Table 1. Significant differences were detected between the ‘Kyuuraku No. 2’ AA genotype and ‘Morioka No. 1’ aa CS-ACS2 genotype. These results suggest that CS-ACS2 may be associated with the monoecious sex phenotype and that CS-ACS2 may be one of several genes that may control the expression of this trait. Since the expression monoecious sex phenotype is a quantitative, we think that CS-ACS2 may be a good candidate for use as a selectable marker for capture of monoecious sex phenotypes with variable expression. However, its action in multiple stress environments (e.g., heat, light & water stress) has not been studied. Such experiments would provide information on the modifying effect of other genes on CS-ACS2.
Figure 1. Southern blot hybridization of ‘Kyuuraku No. 2’, ‘Morioka No. 1’ and theF2 population. Total DNAs were digested with Sac I and electrophoresed on a 0.8% agarose gel. The blot was probed with CS-ACS2. K and M indicate ‘Kyuuraku No. 2’ and ‘Moroioka No. 1’ respectively. Arrows indicate RFLP bands where the marker is λ -Hind III.
Table 1. Number of female flowers of ‘Kyuuraku No. 2’ (A), ‘Morioka No. 1’ (a), F1 hybrid and in each CS-ACS2 genotype in F2.
Genotype of CS-ACS2 in F2 |
||||||
Character |
‘Kyuuraku No. 2’ |
‘Morioka No.1’ |
F1 (K x M)y |
AA |
Aa |
aa |
| No. of female flowers | 2.9 +0.4 | 11.4 + 0.5 | 3.8 + 0.5 | 3.7 ax | 5.9ab | 7.5b |
| No. of plants | 8 | 8 | 8 | 19 | 39 | 21 |
z Seventeen nodes examined per plant.
y K (AR) and M (aa) show ‘Kyuuaraku No. 2’ and ‘Morioka No. 1’, respectively.
x Means followed by the same letter do not differ at 5% significance level by Fisher’s LSD-method.
Literature Cited
- Trebitsh, T., J.E. Staub and S. O. Sharman. 1997. Identification of a 1-aminocyclopropant-1-carboxylicacid synthase gene linked to the Female (F) locus that enhances female sex expression in cucumber. Plant Physiol. 113:987-995.
- Kamachi, S., H. Sekimoto, N. Kono and S. Sakai. 1997. Cloning of a cDNA for a 1-aminocyclopropane-1-carboxylate synthase that is expressed during development of female flowers at the apices of Cucumis sativus L. Plant Cell Physiol. 38:1197-1206.
- Mizusawa, H., N. Hirama and S. Matsuura. 1998. Markers linked to agronomic traits of cucumber (Cucumis sativus L.). Proc. Cucurbitaceae ’98 pp. 366-369.
- Murrey, M.G. and W. F. Thompson. 1980. Rapid isolation of high molecular weight plant DNA. Nucleic Acid Res. 8:4321-4325.