Cucurbit Genetics Cooperative Report 9:12-14 (article 4) 1986
Lawrence K. Pierce, Vicki J. Pierce and Leonard M. Pike
Horticulture Department, Texas A&M University, College Station, TX 77843
In many regions of the world. horticulturalists have to cope with poor water quality, particularly high salt levels which are damaging to many species. Affected by this is the cucumber breeder who is concerned with taking field cuttings from gynoecious selections and rooting them for greenhouse seed production. Fortunately, Cucumis sativus L. has the ability to root fairly easily, but when subjected to saline water, the cuttings may be damaged.
Probably the most common rooting technique is the use of a misting system, either continuous or intermittent. However, where salt levels are a problem, survival and rooting of the cuttings may be impaired. Since large volumes of water are required, use of distilled water is impractical.
Another method employed the initiation of root formation in aerated water (1). Here distilled water is a reliable water source, but quite often this is a slower technique and associated with higher mortality rates. Its primary limitation is in getting rooted cuttings to survive the initial water to soil transition. For these reasons, a very simple and inexpensive technique was developed for rooting cuttings. It provides a rapid field to greenhouse cycling of selected plants because cuttings are rooted directly in a soil medium, and because it requires a small amount of distilled water,
Several 3 x 8 x 1.5 foot (w x 1 x h) chambers were constructed for rooting cuttings. These were made with 2 x 2 inch yellow pine and covered with 6 mil greenhouse plastic (Fig. 1). Each chamber rested on a stainless steel table with a 0.75 inch drain hole in the center. The table top sloped toward the drain. A 10-bulb VHO fluorescent light bank operated by a 24 hr timer was suspended 6 inches above each chamber to give a 12 hr photoperiod. the external chamber temperature was maintained at 19+1°C while the internal temperature fluctuated between 23°C while the lights were on and 19°C when they were off. This provided an ideal diurnal regime associated with beneficial rooting of many species.
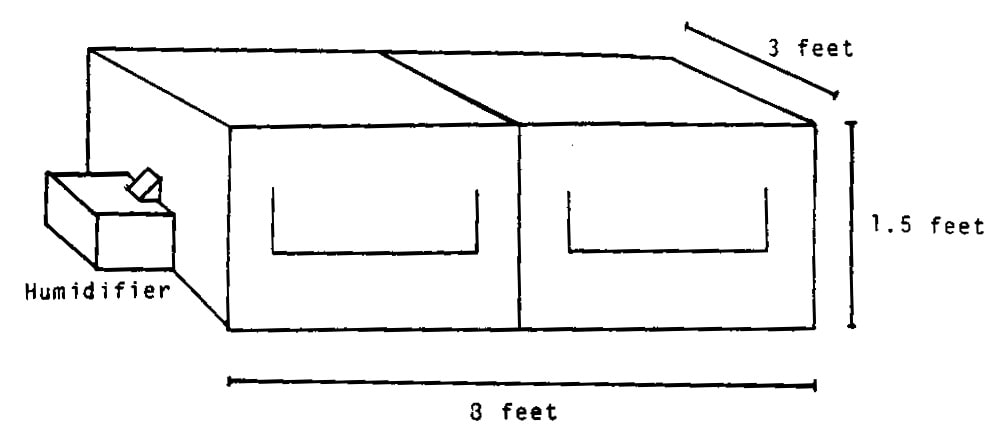
Figure 1. Chamber for rooting cucumber cuttings.
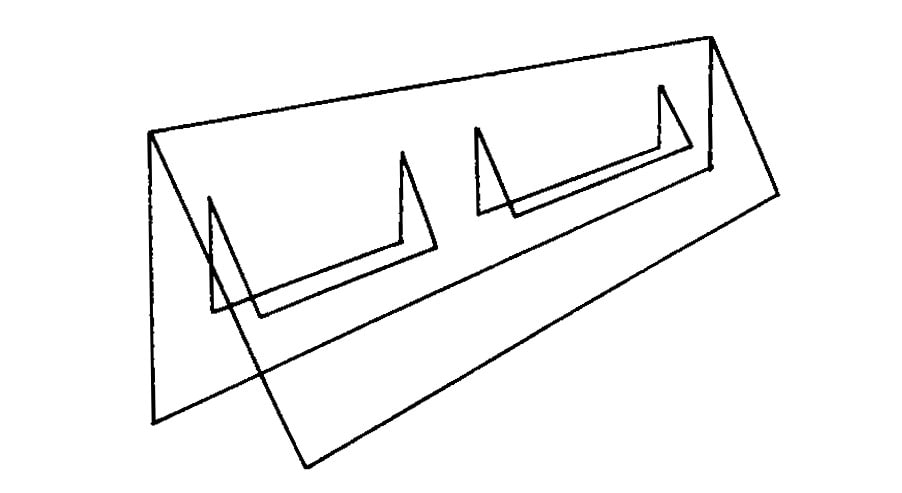
Figure 2. Double plastic flap on front of chamber.
Side port cool mist humidifiers were located at each end of the chambers with their ports penetrating the chamber walls. These humidifiers operated on a second 24 hr time clock, and utilized distilled water to create a high relative humidity without salt buildup in the humidifier. The table top drain allowed excess condensed water to escape, while a double plastic flap system was used in front to keep excess humidity from escaping (Fig. 2). Soapy water was sprayed between these two plastic layers in order to maintain a good seal.
The humidifiers were run continuously until 96 + 1% relative humidity was reached. Then the timers were set to allow the humidifiers to cycle on and off occasionally for maintenance of this level. The total operating time varied with different brands. It was best to have a matched set if a single chamber was to maintain a uniform humidity pattern. Adjustments were made to temporarily decrease humidity levels if disease became apparent.
Selections were sprayed in the field with SADH (2) one week prior to cutting harvest. At harvest, cuttings five or six nodes long were taken from the most stocky terminals and dipped into a mixture of Banrotz (1.1gm/gal) and Agrimyciny (0.4gm/gal) for 5 min. The cuttings were then trimmed to 3 or 4 nodes with all leaves left intact. They were inserted with 3 inch square peat pots containing artificial media saturated with distilled water. These pots were then placed in the chambers on top of inverted flats with a 1 inch spacing between each pot. This maximized air circulation and decreased the rate of disease spread if it occurred. The inverted flats allowed the pots to be off the cool, damp table surface.
Once roots began to emerge through the pots, they were moved to a light bank at 23°C and normal room humidity for 3 to 5 days for a hardening period. A single fertilizer application was made at that time. After this, they were transplanted to larger pots in a greenhouse. A full fertilizer feeding program then brought them to normal growth in one to two weeks.
This system had several advantages. There was a relatively rapid turn-around period involved. It minimized the amount of distilled water required. Disease could be controlled fairly easily. Rooting actually took place within a soil medium, so transplant shock was minimized and an 85% survival rate was achieved. This technique may also be useful for the propagation of other cucurbit species.
Literature Cited
- Foster, R.E. 1963. Aeration, light and type of cutting for vegetative propagation of muskmelon (Cucumis melo L.). Proc. Amer. Soc. Hort. Sci. 83:596-598.
- Shehata, M.A., D.W. Davis and P.E. Read. 1984. Vegetative propagation of cucumber. HortScience 9(6):575-576.
z Supplied as “Banrot”, a 40% wettable powder; product of Mailindckrodt Inc.
y Supplied as “Agri-mycin 17” with 21.2% streptomycin sulfate; product of Pfizer Inc.