Cucurbit Genetics Cooperative Report 10:11 (article 12) 1987
Staub, J. E. and L. Crubaugh
U. S. Department of Agriculture, Agricultural Research Service and Department of Horticulture, University of Wisconsin, Madison, WI 53706
It has long been clear that environmental factors affect cucumber (Cucumis sativus L.) fruit development (6), and sex expression (1,3,8). In a study by Lower et al. (4) plant density had little effect on the number of pistillate flowers in a series of gynoecious inbreds, but increased density reduced pistillate flowers in several gynoecious (G) x monoecious (M) hybrids made with ‘Gy14-1’. Increases in staminate flower production were observed with increasing densities in several G x M F1 hybrids. Under more controlled environmental conditions no pistillate flowers were present on determinate monoecious plants under a 9 hrs. photoperiod at high temperatures or on indeterminate monoecious plants at longer photoperiods (12 hrs.) and high temperatures (5). In studies by Cantliffe (2) a series of gynoecious processing cucumber cultivars produced more staminate flowers at a light intensity of 17,200 lux than at 8,600, 12,900 or 25,800 lux, while a gynoecious inbred line (MSU 713-5) was unaffected. In contrast to plants grown at a constant temperature of 16°C, those grown at 30°C produced some staminate flowers.
The USDA cucumber breeding program has made use of the hermaphroditic (H) character in cucumber in an attempt to improve gynoecious sex stability. It was concluded that there are no differences in fruit quality or yield among GxG and GxH hybrids produced by genetically similar inbred lines (7). However, because a critical sex expression test has not been devised, it has not been determined whether the hermaphrodite character in these G x H hybrids provides increased sex stability. Therefore, a study was designed to identify environmental stresses which could be used to demonstrate whether sex stability differences exist among these G x G and G x H hybrids.
The imposed stresses included a cool temperature shock, “inadequate” fertilizer concentrations, and sand used as a growing medium. Five genotypically different plants subjected to these conditions and grown under greenhouse conditions were compared to those grown at higher temperatures (25 to 30°C), in a standard greenhouse soil medium, and having received “adequate” fertilization. The factorial set of treatments (5 genotypes x 2 temperatures x 2 fertilizations x 2 growing media) were evaluated in a completely randomized design with 4 replications for plant weight, days to anthesis, number of staminate flowers, and number of pistillate flower abortions in the first 10 nodes. Seven plants in a pot (20 cm in diameter) were considered the experimental unit. The genotypes used to evaluate differences for gynoecious sex stability were two gynoecious inbreds (WI 1379 and WI 1983) and 3 hybrids [1983G x 1379H, 1983G x 1379G, and 1983G x 1379A (andromonoecious)].
Cool shocking of plants was accomplished by subjecting a portion of the plants at the third true leaf stage to 4°C for 8 hrs. under darkened conditions. Plants grown in sand and designated as “adequate” fertilizer, received a standard fertilizer mixture (GEWA proportioner delivering 1.35 kg Peter’s 20-20-20, 0.45 kg urea and 56 gm Peter’s micronutrient) to saturation at 4 day intervals beginning 8 days after sowing. Those assigned the “inadequate” fertilizer treatment received the same applications, except for the last 3 watering periods where they received consecutively 50, 25 and 25% of full strength fertilizer. Plants grown in greenhouse mix (1 sand:2 soil:1 peat:1 perlite, by volume) receiving “adequate” fertilizer were treated initially 14 days after sowing and then weekly thereafter, while those designated as “inadequate” fertilizer treatments were given 50, 25 and 25% of full strength during the last 3 waterings. Treatments were chosen such that plants growing under the imposed stress treatments reached anthesis, but were visually stunted.
Significant differences were detected among the main effects: fertilization, media type, and genotype (Table 1). Amount of fertilizer application effected average plant height, and differences in average plant height, days to anthesis, and percentage of staminate flowers and pistillate abortions were produced by the affects of the growing media used. In addition, genotypic differences were observed for all traits measured. Significant 2 and 3-way interactions were not detected, except between soil and genotype for days to anthesis and percentage of staminate flowers.
Means and LSD’s for these main effects are provided in Table 2. “Inadequate” fertilization and growth of plants in sand reduced the average plant weight and percentage of pistillate abortions, but increased days to anthesis and the percentage of staminate flowers. The inbred line WI 1379G was on the average smaller, and WI 1983G flowered later than other genotypes. While the percentage of staminate flowers was highest in WI 1983G, the percentage of pistillate abortions was highest in the hybrid 1983G x 1379H. Correlation coefficients (Table 3) indicate that a moderately positive association exists between percentage of pistillate abortions and average plant weight, and a lack of association between average plant weight and days to anthesis. Moreover, a negative association was also detected between days to anthesis and percentage of pistillate abortions.
These data suggest that although the “inadequate” fertilization schedule employed produced smaller plants, it did not affect the sex expression of those plants. In contrast, the sand media produced changes in sex expression, and therefore might be useful treatment in future studies. The hermaphroditic character in the cross 1983G x 1379H provided the most stabilizing effect on sex expression of the crosses using 1983G as the maternal parent. Although these results are equivocal, they suggest that the hermaphrodite character may be potentially useful in stabilizing the gynoecious sex expression under environmental stress conditions used in this study.
Table 1. Mean squares for average plant weight, days to anthesis, percentage of staminate flowers, and pistillate flower abortion of 5 cucumber (Cucumis sativus L.) genotypes grown in 2 soil media, under 2 types of fertilization after cold shock at the third true leaf stage.
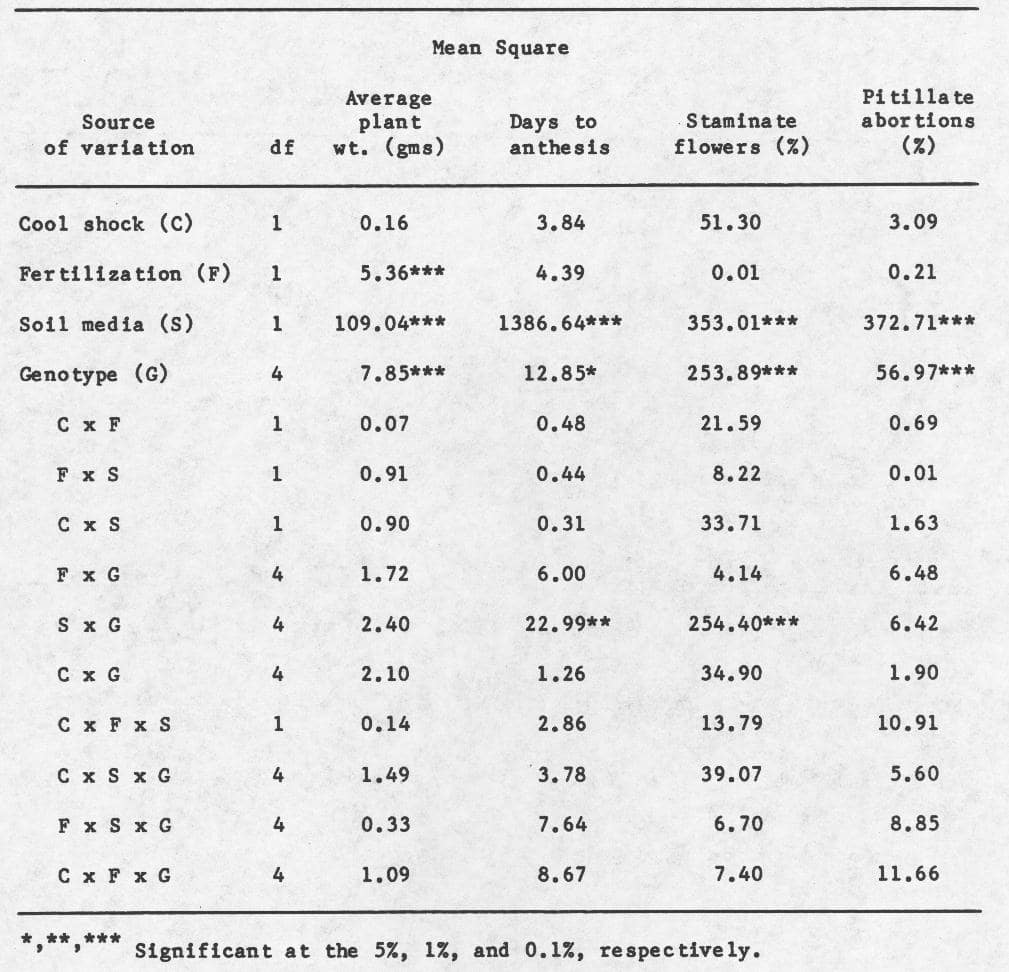
Table 2. Means and LSD’s of significant main effects (temperature, fertilization, soil media and genotype) for morphological traits of cucumber (Cucumis sativus L.) genotypes grown under several environmental conditions.
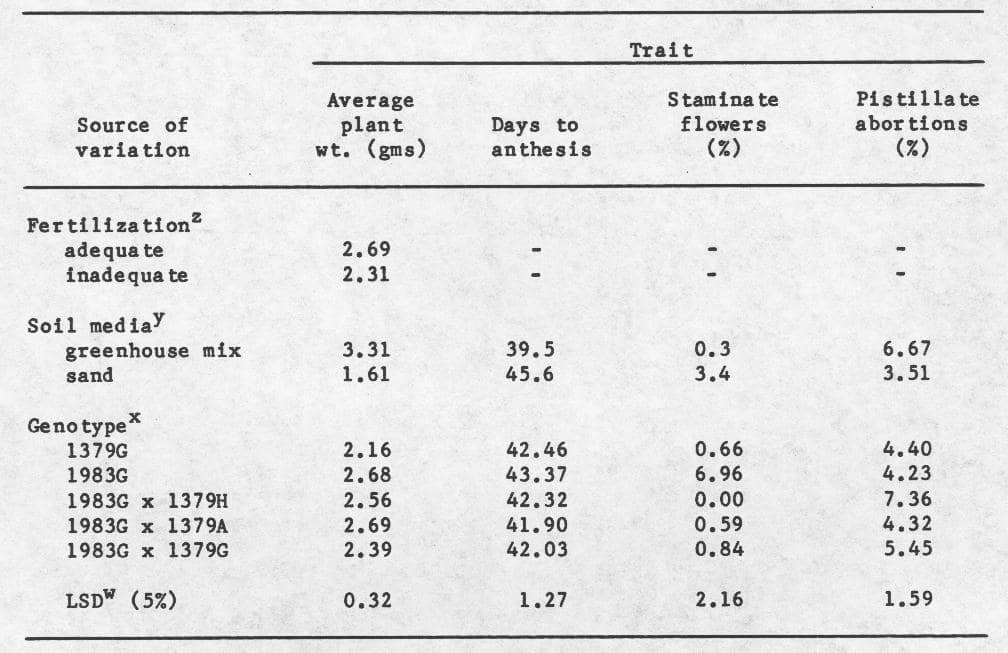
z Mixed for GEWA proportioner at 1.35 kilograms Peters 20-20-20, 0.45 kilograms urea & 56 gms Peter’s micronutrient.
y Greenhouse media contains equal amounts of a 1 sand:2 soil:1 peat: 1 perlite (by volume) mix.
x G & H indicate the inbred line is gynoecious and hermaphroditic, respectively.
w Mean comparison for genotype.
Table 3. Phenotypic correlation coefficients of morphological traits of 5 cucumber (Cucumis sativus L.) genotypes grown under several environmental conditions.
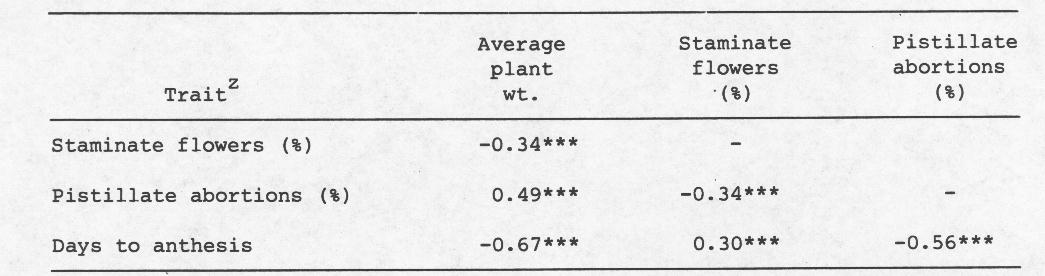
z Data were merged over growing conditions since scatter plots of data were randomly distributed.
***Significant at 0.1% probability level.
Literature Cited
- Atsmon, D. and E. Galun. 1962. Physiology of sex in Cucumis sativus (L.). Leaf age patterns and sexual differentiation of floral buds. Ann. Bot. 26:137-146.
- Cantliffe, D.J. 1981. Alteration of sex expression in cucumber due to changes in temperature, light intensity, and photoperiod. J. Amer. Soc. Hort. Sci. 106:133-136.
- Edmond, J. 1930. Season variation in sex expression of certain cucumber varieties. Proc. Amer. Soc. Hort. Sci. 27:329-332.
- Lower, R.L., O.S. Smith, and A. Ghaderi. 1983. Effects of plant density, arrangement, and genotype on stability of sex expression in cucumber. HortScience 18:737-738.
- Lower, R.L., J.D. McCreight, and O.S. Smith. 1975. Photoperiod and temperature effects on growth and sex expression in cucumber. HortScience 10:318 (Abstr.).
- Miller, C.H. and S.K. Ries. 1958. The effect of environment on fruit development of pickling cucumber. Proc. Amer. Soc. Hort. Sci. 71:475-479.
- Staub, J.E., B. Balgooyen, and G.E. Tolla. 1986. Quality and yield of cucumber hybrids using gynoecious and bisexual parents. HortScience 21:510- 512.
- Tiedjens, V.A. 1928. Sex ratios in cucumber flowers as affected by different conditions of soil and light. J. Agr. Res. 36:721-746.