Cucurbit Genetics Cooperative Report 10:93-99 (article 51) 1987
O. Shifriss
21 Walter Ave., Highland Park, NJ 08904
Interspecific hybridization (11). The cross C. pepo x C. moschata is important from both theoretical and practical points of view. The natural reproductive isolation between these species is not as strong as that between other species of Cucurbita. Therefore, it might be possible to identify some of the major genetic barriers which keep these species apart. Furthermore, this cross can result in gene exchanges of economic value. Of special significance is the fact that, unlike the extreme susceptibility of C. pepo to cucurbit pests, C. moschata is an excellent source of resistance. Unfortunately, my notes on this cross are fragmentary.
In 1947 1 obtained hybrid seed from crossing a bush line of ‘Table Queen’ (C. pepo) and ‘Butternut’ (C. moschata), using the former as seed parent. The cross was made at Fordhook Farms (W. Atlee Burpee Co.), Doylestown, PA, early in the morning (5-6 AM) of a cloudy fall day. The 20 cross-pollinated flowers of ‘Table Queen’ developed into mature fruits and all of them contained viable hybrid seed, ranging from 3 to 25. Parenthetically, an earlier report (2) suggested that ‘Table Queen’ may not be compatible with C. moschata. My results support the conclusion of some other breeders that favorable environmental conditions are essential for successful hybridization. The F1 plants were large, semi-bush, and vigorous. The average fruit weight of the parents was 700g (Table Queen) and 1000g (Butternut) and that of the F1, 5000g, a remarkable heterosis for fruit size in squash. The F1 fruit had a large central cavity and the “flesh” was of poor quality. The F2 consisted of many male-female sterile segregates which exhibited serious growth abnormalities.
Some of the consequences of a similar cross were observed during 1979-1983. This cross was between ‘Jersey Golden Acorn’, BB, bearing golden, acorn- shaped fruits (C. pepo), and ‘Burpee Butterbush’, B+B+, bearing tan, bottle- shaped fruits with short thick “neck” (C. moschata). The fruit of the F1, BB+, was bicolor and intermediate in shape. The average fruit of the parents was 680g and 700g respectively, and that of the F1, 1041g. The F2 (n = 283) was highly variable in fruit characteristics, in degree of fertility, and perhaps in proneness for parthenocarpy. With respect to fruit weight, the F2 ranged from 146g to 2080g. But the most conspicuous features of the F2 were severe growth abnormalities. Three other comments are of interest. First, the BC1, F1, BB+, x ‘Jersey Golden Acorn’, BB, consisted of a few BB plants which produced deeply scalloped disk-shaped fruits (similar to those of ‘White Bush Scallop’ of C. pepo) of intense golden color. Second, some F2 segregates produced fruits of exceptionally thick flesh and very small central cavity. Third, some segregates were male-female sterile; others were male sterile early in plant development and male fertile later on; and still others were self-incompatible, but cross-compatible with ‘Jersey Golden Acorn’ as seed parent.
There is little doubt that the cross C. pepo x C. moschata deserves critical analysis as well as use in breeding. But it is evident that the ease by which F1, F2, and backcross seed can be obtained does not reflect the degree of ‘disharmony” between the two genomes.
Intraspecific hybridization. Each of the economically important species consists of several distinct groups of cultivars. Among other things, these groups differ from one another in fruit characteristics and duration of their artificial or geographical isolation. The short-term concerns in the squash seed industry are to perpetuate the existing groups of cultivars and to develop F1 hybrids from intra-group crosses. In the long-term, it would be advisable to utilize the advantages of inter-group crosses. First the F1 hybrid of inter-group crosses often exhibit greater heterosis than F1 hybrids of intra-group crosses. However, the development of such hybrids will require additional breeding efforts. Second, some inter-group crosses generate tremendous genetic diversity without sterility and growth abnormalities. These crosses will provide the breeder an access to a larger portion of the gene pool including untapped potentially useful genetic elements. It is expected that most of this diversity will be inferior. But some genotypes will exhibit new useful traits; others will enhance the economic value of known desirable traits; and still others will reduce the expression of undesirable characteristics. This thought received its impetus from studies of genes B and M as well as from studies of growth habit, flowering, and sex expression.
Effects of B. This gene is widespread in C. maxima. A gene of similar behavior was transferred from the bicolor ornamental gourds to some of the major edible cultivars of C. pepo (7). In 1980, the B genes of C. maxima and C. pepo were transferred to C. moschata (9). And more recently, Paris and his colleagues in Israel reported an additional B transfer, from C. pepo and C. moschata.
Gene B conditions precocious chlorophyll depletion in fruit and this leads to precocious yellow pigmentation. Furthermore, B can bring about precocious depletion of chlorophylls in all other normally photosynthetic organs. In a broader sense, B can exhibit many “secondary effects” some of which are deleterious and others, beneficial. There is growing evidence indicating that the secondary effects result from interactions between B and other elements in the gene pool, and that the detrimental and beneficial effects are separable in breeding operations. Some of these elements are responsible for distinct phenotypes of their own, but most of them are invisible phenotypically except through their interactions with B. These “invisibles” are difficult to identify. Indeed, very few valuable invisibles (modifiers or regulators) have been identified (8, 10). Therefore, in cases in which the invisibles are not identified, the beneficial effects of B are surmised from comparison between isogenic or near-isogenic lines, BB and B+B+, of different backgrounds. But incisive tests are often lacking.
There exists a wide range of variation in the levels of fruit carotenoids among standard, B+B+, cultivars of Cucurbita. Previous observations (1956- 1962) of the B effect on fruit color in C. pepo suggested that this gene can increase the carotenoid content, but that increases of large magnitudes can be achieved through interactions between B and genes for dark green fruit color such as L, and provided gene inhibitors are not present (7). Subsequent chemical analyses by S. A. Garrison (in reference 6) and others (Table 1; see also reference 5) essentially confirmed this suggestion. Similar interactions exist in C. maxima and C. moschata.
The mechanism governing the synthesis of different fruit carotenoids is not understood. The specific role of environmental factors is not known. And most of the genes (including enhancers and inhibitors) which control pigmentation have not been identified. Weak or strong inhibitors appear to act a few days after B. They affect either the external portions of the fruit or both. They study of Schaffer et al (4) and other observations pose a number of intriguing questions concerning the role of B on plastid transformation in different tissues. It seems that a product of B, perhaps a diffusible substance (7), is the signal that regulates plastid transformation.
The first commercially available carotenoid-rich cultivar of summer squash was Burpee Golden Zucchini. It was developed by T. C. Torrey and introduced in 1973. This cultivar is similar to our PFZ (Precocious Fordhook Zucchini) breeding line developed in 1963. Both lines originated from the same germplasm. The use of cross IL-B x NJ-B (9) in breeding could lead to the development of nutrient-rich BB cultivars adapted to mechanized harvesting. Except for their green leaf blades, all other plant parts of these cultivars would be precociously golden. Such cultivars might be valuable to the food processing industry for various purposes, including dehydrated meal; stems for feed supplement, and fruit and seeds for human consumption.
In summer squash, the fruits of several BB lines have firmer flesh textures and exhibit new pleasing flavors to the extent not found in any of the known standard B+B+ cultivars. Thus, gene B could be valuable for the development of new cultivars that are better adapted for use in freezing. ‘Blondie’ is the first BB+ hybrid designed specifically for this purpose (David Groff, personal communication). The fruits of some “precocious” winter squash, such as ‘Jersey Golden Acorn’, BB+ have a flavor reminiscent of yellow sweet corn. This is a new incipient trait which might be greatly reinforced through incorporation of genes for higher sugar content and absence of remnant of bitterness. I see the possibility for the development of dual-purpose B cultivars of high quality in C. pepo and C. maxima. These cultivars will serve both as “summer” and “winter” squash. And among them there will be cultivars used in food as water chestnut.
In 1966, 1 suggested that gene B can enhance female expression of the background of one of the summer squash cultivars. Since then this effect of B has been confirmed in repeated comparative tests of isogenic lines, BB and B+B+, of Early Prolific straightneck background. Results of one of these tests are presented in Table 2. The BB line involved is known as PEP. It was distributed among many breeders and would be helpful if some of them publish their own findings on this subject. PEP is one of the parents of “multipik”, a hybrid known for its strong female expression. As far as I know, the most strongly female line among the monecious cultivars of Cucurbita is inbred NJ260, BB. Yet, the effect of B on female expression was not reported or has not been clearly evident in other backgrounds.
The recently synthesized closely related gynoecious lines (U.S. Patent pending), BB and B+B+, are late-flowering and 100% female in some environments, but they differentiate varying proportions of male flowers in other environments. Although we have not identified all the non-genetic factors which may promote late-flowering and male expression, it is clear that high temperatures favor both. The fact that B was not essential for the synthesis of these female lines does not necessarily exclude the possible role of B as an accelerator of pistillate flower differentiation in some backgrounds.
One of the advantages of the new gynoecious lines is that they can be used in strongly female monoecious cultivars which branch and differentiates their pistillate flowers more or less simultaneously, a desirable trait for both manual and mechanized harvesting.
Although gene B is a highly stable element in some backgrounds, it originated through the consequences of nuclear instability and is prone to instability in some other backgrounds. Its behavior recalls the behavior of a mobile element. This gene can manifest at least 2 kinds of variegation: one kind appears as a developmentally fleeting, imprecise, and unpredictable pattern seemingly due to phenotypic plasticity; and the other kind appears as a precise and predictable pattern due to mutation of B. Fruits, leaves and other parts of the plant may be affected. As a result, B is a potential source of variations some of which may be valuable in breeding of both edible and ornamental cultivars.
Finally, according to my observations all the known deleterious effects of gene B can be supressed without interference on the expression of its beneficial effect. These observations support the following hypothesis. A potentially deleterious gene can become beneficial if two requirements are fulfilled. First, the gene must have one or more beneficial effects. Second, the gene pool must carry elements which supress the deleterious effect independently of the beneficial one.
Virus infection. Some late-maturing pumpkins of C. pepo possess mild field tolerance to virus infection. In addition, the extremely intense dark green foliage of some breeding lines is more tolerant to virus infection than their fruit, particularly golden fruit.
The implication of gene B in fruit tolerance to the adverse effects of virus infection is difficult to assess. The best documented case is that of ‘Multipik”, introduced in 1981. According to T. H. Superak (personal communication), ‘Multipik’, BB is near-isogenic to ‘Golden Girl’, B+B+, but the former is less adversely affected by CMV-induced fruit symptoms than the latter. In addition, Adlerz et al (1) reported that the fruits of ‘Multipik’ are partially tolerant to WMV-2. And Paris et al (3) noted fewer virus- induced fruit symptoms (presumably CMV and WMV-2) in ‘Goldy’, BB+, than in ‘Gold Rush’, BB+. Obviously, if B is a contributing factor it alone cannot account for the difference in response between these hybrids.
Another issue worthy of further exploration is the possible escape mechanism of the silvery-leaf fruit trait against aphids (see communications on this subject in previous issues of CGC Rpt.). It is known that silvery leaves reflect more light than green leaves. And the inference is that greater light reflection tends to repel aphids. Our first silvery line was NJ260, BB. This line, however, proved to be be highly susceptible to wilting under mild water stress conditions in the field. We transferred the genotype for the silvery trait to ‘Precocious Caserta’, BB, and found that the resulting silvery line, BB, is not more susceptible to wilting than standard B+B+, green cultivars.
The full genotype of the silvery trait has not been identified. The hypothesis is that it consists of gene M, for leaf mottling, and several modifiers. The silvery phenotype greatly fluctuates, in response to non- genetic variations, from different degrees of mottling (silvery spots) to uniformly silvery leaves. Attempts should be made to stabilize this trait through crosses between silvery lines which exhibit developmentally different modes of expressions.
Future direction. The long range goal in squash breeding is to restructure the Cucurbita genome through interspecific and intraspecific crosses for the purpose of transforming squash into a staple food crop of worldwide distribution.*
Table 1. Levels of carotenes and xanthophylls in ripe fruits of B+B+ and BB inbreds of four cultivar backgrounds of Cucurbita. Each determination was based on a composite fresh sample obtained from the mesocarp of ten fruits. All inbreds were grown in a replicated test. From field studies, New Brunswick, New Jersey, 1978.
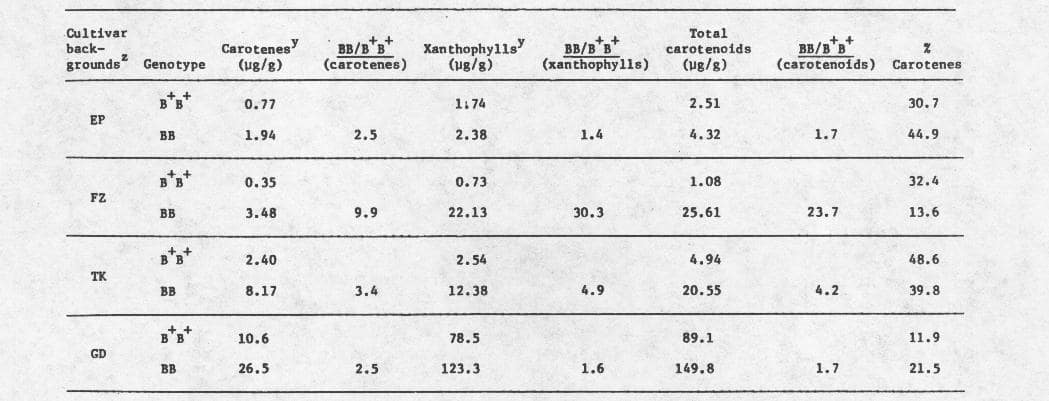 z Key to cultivar backgrounds: EP =’Early Prolific Straightneck’, FZ – ‘Fordbook Zucchini’, TK = ‘Table King’, all three of Cucurbita pepo; GD =’Golden Delicious’ (Munger’s strain) of Cucurbita maxima. B+B+ and BB inbreds of GD may not be entirely isogenic.
z Key to cultivar backgrounds: EP =’Early Prolific Straightneck’, FZ – ‘Fordbook Zucchini’, TK = ‘Table King’, all three of Cucurbita pepo; GD =’Golden Delicious’ (Munger’s strain) of Cucurbita maxima. B+B+ and BB inbreds of GD may not be entirely isogenic.
y Determinations were made by the New Jersey Feed Laboratory, 910 Pennsylvania Avenue, Trenton, NJ 08603, based on AOAC procedure.
Table 2. Female expression in B+B+ and BB inbreds of ‘Early Prolific Straightneck’ background. The data are based on a replicated test of 10 plants per inbred. From field studies, New Brunswick, New Jersey, 1976.
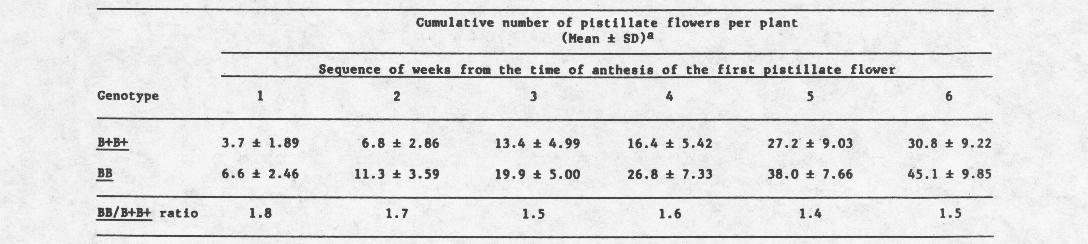 a The difference in cumulative number of pistillate flowers between BB and B+B+ plants is statistically significant at each of the six weeks (P<0.01).
a The difference in cumulative number of pistillate flowers between BB and B+B+ plants is statistically significant at each of the six weeks (P<0.01).
Literature Cited
- Adlerz, W. C., G. W. Elmstrom. and D. E. Purcifull. 1985. Response of ‘Multipik’ squash to mosaic virus infection. HortScience 20:892-893.
- Erwin, A. T. and E. S. Haber. 1929. Species and variety crosses in cucurbits. Bulletin No. 263, Iowa Agricultural Experiment Station, Ames, Iowa.
- Paris, H. S., Z. Karchi, H. Nerson and Y. Burger. 1983. Yield and yield quality in precocious yellow zucchini cultivars. HortScience 18:724-726.
- Schaffer, A. A., C. D. Boyer and T. Gianfagna. 1984. Genetic control of plastid carotenoids and transformation in the skin of Cucurbita pepo L. fruit. Theo. Appl. Genet. 68:493-501.
- Schaffer, A. A., H. S. Paris and I. M. Ascarelli. 1986. Carotenoid and starch content of near-isogenic B+B+ and BB genotypes of Cucurbita. J. Amer. Soc. Hort. Sci. 111:780-783.
- Shifriss, O. 1974. Manifestations and use of gene B in Cucurbita. In the proceedings of the 19th International Horticultural Congress. Vol. 1, page 100. Warsaw.
- Shifriss, O. 1981. Origin, expression and significance of gene B in Cucurbita pepo L. J. Amer. Soc. Hort. Sci. 106:220-232.
- Shifriss, O. 1982. Identification of a selective suppressor gene in Cucurbita pepo L. HortScience 17:637-638.
- Shifriss, O. 1986. Relationship between the B genes of two Cucurbita species. Cucurbit Genet. Coop. Rpt. 9:97-99.
- Shifriss, O. and H. S. Paris. 1981. Identification of modifier genes affecting the extent of precocious fruit pigmentation in Cucurbita pepo L. J. Amer. Soc. Hort. Sci. 106:653-660.
- Whitaker, T. W. and G. N. Davis. 1962. Cucurbits. Interscience Publications, Inc., New York.
* Some of the above notes, including tables 1 and 2, were taken from a six year old manuscript originally written for a cucurbit book whose publication has been delayed.