Cucurbit Genetics Cooperative Report 11:64-67 (article 27) 1988
Love, S.L.
U. Idaho Aberdeen Research and Extension Center, Aberdeen, ID 83210
Rhodes, B.B.
Clemson U. Edisto Research and Education Center, Blackville, SC 29817
This research was supported by a grant from the USDA CRGO.
Previous reports (1, 3, 6, 7) indicate that resistance to Colletotrichum lagenarium (Pass.) Ell. and Halst. in watermelon is governed by a single dominant gene. These studies differed in classification of resistance, race identification, inoculum levels and susceptibility of greenhouse-grown seedlings.
If a single gene controls resistance to race 2, it should be simple to incorporate the high level of resistance found in resistant into commercially accepted cultivars. Actually, no cultivar has been developed with as much resistance to race 2 anthracnose as the resistant source. A greenhouse study of inheritance to race 2 resistance in documented resistant and susceptible Citrullus genotypes was conducted in 1982. A field study was conducted in 1983. Methods were modified in the second study as indicated.
Methods. An isolate of the fungus was obtained from the EREC and determined to be race 2 by the method of Jenkins et al. (2). Culture and spore production methods were similar to those of Littrell and Epps (4). Randomized blocks of parents and progeny of the host were mist inoculated with spore suspensions: 50,000 spores/ml in the greenhouse and 20,000 spores/ml in the field study. Greenhouse seedlings were inoculated at the 2-4 leaf stage. Field plants were inoculated at fruit set. Greenhouse seedlings were rated 8 days after inoculation on a 0-10 scale (0 = no lesions, 10 = dead) by comparing with a set of 11 plants representing 1 point on the scale. Plants rated 0-6 were considered resistant, 7-10 susceptible, Field plants were rated in two ways five weeks after inoculation: 1 – the oldest branch on each plant was rated for percent defoliation and 2 – percent of the remaining leaves showing lesions. A disease index was a composite of both ratings. Plants indexed 0 – 85 were considered resistant, above 85 susceptible,. The average between resistant and susceptible parent means was used as the division between resistant and susceptible plants.
Treatments assigned to each block were F1, F2 and BC1 generations from a diallel cross among the resistant watermelon plant introduction PI 189225, PI 299379, the susceptible watermelon cv, New Hampshire Midget and a resistant line of Citrullus colocynthis (1) Schrad., designated R309.
Results. The greenhouse seedling study could not distinguish resistant plants from susceptible plants. Anthracnose symptoms developed on even the most resistant lines. This result is consistent with the report of Winstead et al. (7), who reported that PI 189225 was susceptible to anthracnose race 2 and Sowell et al. (5), who reported the PI resistant. Unlike Sowell et al., Winstead et al. depended entirely on greenhouse seedling inoculations to screen for resistance and used an inoculum level of 50,000 spores/ml instead of 20,000 spores/ml.
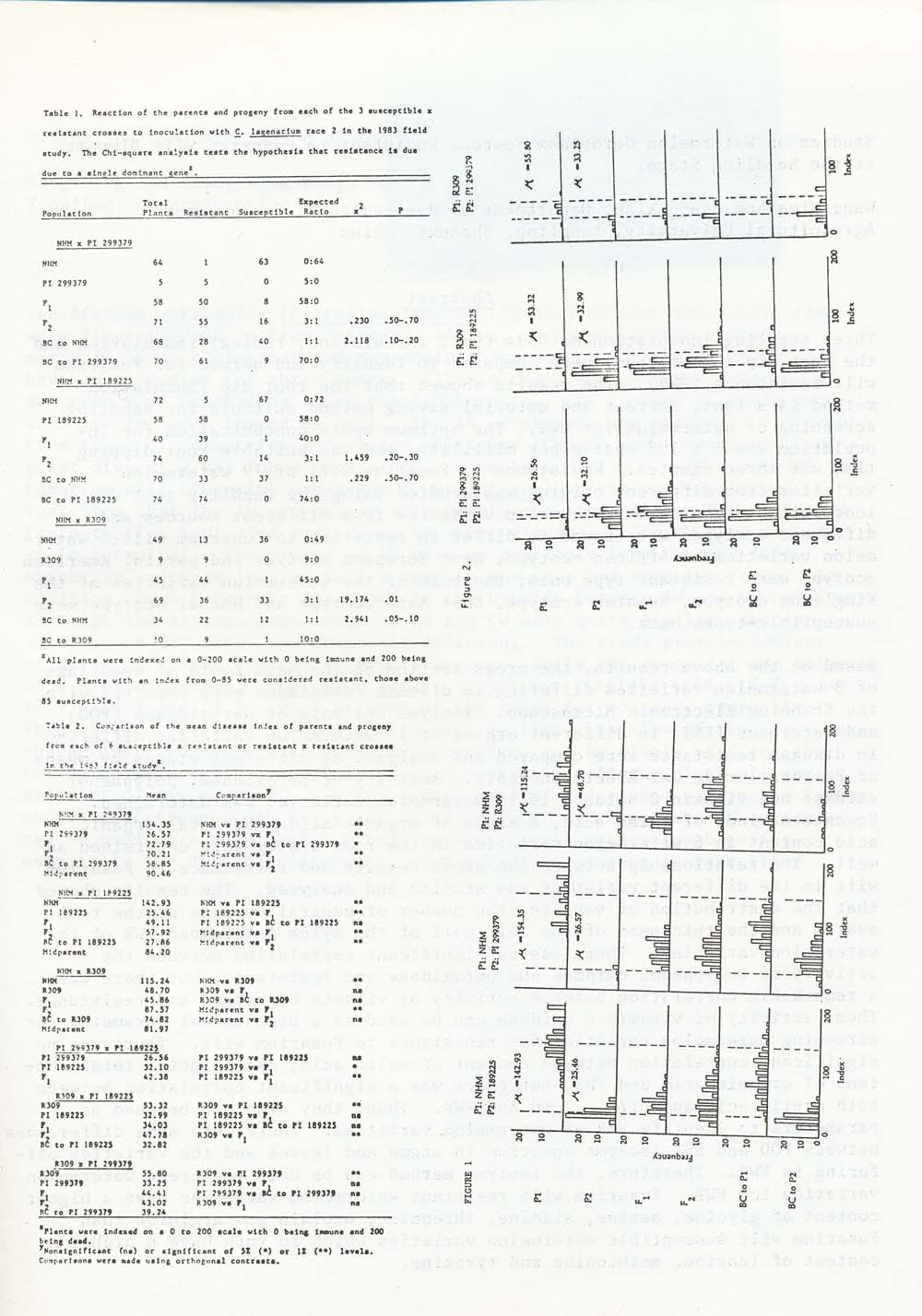
Resistant and susceptible plants were more easily identified in the field study. Table 1 shows the ratios of resistant and susceptible plants for each of the 3 susceptible x resistant set of parent-progeny populations. Chi-square analysis of F2 and backcross progenies of the NHM x PI 299379 and NHM x PI 189225 crosses cannot reject the hypothesis that resistance in PI 299379 and 189225 are controlled by a single dominant gene. However, the NHM x R309 progeny does not fit any expected ratio for a 1 or 2 gene dominant, recessive of additive trait.
Frequency distributions of resistance levels in PI 299379 and PI 189225 and their progeny suggest that the inheritance of resistance is more complex than that suggested by Chi-square analysis. The shift of the F 1 populations toward the susceptible parent does indicate complete dominance. Also, the F2 populations are not divided into discrete classes.
Comparison by means using orthogonal contrasts for each of the 6 sets of parent-progeny populations is found in Table 2. A comparison of the F1 and F2 with the midparent from PI 299379 x NHM supports the conclusion that resistance is largely due to dominance. However, the failure of the F1 and backcross generations to show as much resistance as PI 299379, the lack of a bimodal distribution in the F2 and the failure of the F2 to produce plants as susceptible as the susceptible parent indicates the presence of other modifying genes. The same conclusion can be drawn from the progeny populations from PI 189225 x NHM.
Comparison of the F1 and backcross populations from R309 x NHM to the resistant parent indicates resistance is due to complete dominance. However, the F2 is not significantly different from the midparent and fails to support the conclusion of either 1 or 2 dominant genes. Instead, it is more representative of the distribution expected from several dominant genes acting in concert.
The F1 population from PI 299379 x PI 189225 (Table 2) is significantly more susceptible than either of the resistant parents, indicating the presence of additive or recessive resistance factors in each line that are anot found in the other. The failure of the F2 population to show distinct segregation for susceptible plants suggests that both lines share a common major dominant gene factor for resistance and that the unshared factors were minor modifiers.
Comparison of progeny means from R309 x PI 189225 indicates that the higher level of resistance displayed by PI 189225 is due to dominance. Thus, it follows that R309 owes its resistance to a set of factors separate from PI 189225. The same conclusion can be reached from the comparison of progeny means from R309 x PI 299379. Small populations, due to poor germination, made statistical analysis of this family difficult. Nevertheless, the involvement of separate gfenetic factos was apparent from the segregation of the progeny.
In conclusion, two types of inheritance for resistance were identified in the 3 resistant lines. R309 (C. colocynthis), demonstrated an intermediate level of resistance attributed to several dominant genes acting in concert. In PI 299379 and PI 189225 resistance is controlled largely by a single dominant gene. In the later 2 lines, resistance is modified by minor genes.
Literature Cited
- Hall, C.V., S.K. Dutta, H.R. Kalia and C.T. Rogerson. 1959. Inheritance of resistance to the fungus Colletotrichum lagenarium (Pass,) Ell. and Halst. in watermelons. J. Amer. Hort. Sci. 75:638-643.
- Jenkins, S.F., Jr. N.N. Winstead and C.L., McCombs. 1964. Pathogenic comparisons of three new and four previously described races of Glomerella cinqulata var. orbiculare. Plant. Dis. Rep. 48:619-622.
- Layton, Duke V. 1937. The parasitism of Colletotrichum lagenarium (Pass.) Ell. and Halst. Iowa Agr. Exp. Sta. Bull. 223.
- Littrell, R.H. and W.M. Epps. 1965. Standardization of a procedure for artificial inoculation of cucumbers with Colletotrichum lagenarium.
- Sowell, G., Jr., B.B. Rhodes and J.D. Norton. 1980. New sources of resistance to race 2 anthracnose in watermelon. J. Amer. Soc. Hort. Sci. 105:862-865.
- (Not referenced in text.)
- Winstead, N.N., M.J. Goode and W.S. Barham. 1959. Resistance in watermelon to Colletotrichum lagenarium races 1, 2, and 3. Plant Dis. Rep. 43:570-576.