Cucurbit Genetics Cooperative Report 12:55-57 (article 24) 1989
W.A. Mackay, T.J. Ng and F.A. Hammerschlag
Department of Horticulture, University of Maryland, College Park, MD 20742 USA; Plant Molecular Biology Laboratory, Agricultural Research Service, U.S. Department of Agriculture, Beltsville, MD 20705 USA
Successful in vitro selection requires the regeneration of plants from unorganized tissue. We have previously reported the regeneration of three cultivars of muskmelon (‘Hales Best’, ‘Iroquois’ and ‘Perlita’) using a modification of the protocol described by Moreno et al. (3). However, regeneration with other growth was low for ‘Perlita’ and ‘Iroquois’. Regeneration with other growth regulator combinations has been reported (1, 4). To improve low regeneration efficiency alternate growth regulator combinations were tested.
Surface disinfestation and removal of cotyledons was accomplished as previously described (2). Cotyledons were plated on 25 ml of medium contained in 15×90 mm petri dishes. The basal medium consisted of Murashige and Skoog salts and vitamins, 3% sucrose, 0.8% Phytoagar supplemented with 0.0, 0.5, 1.0, 2.5, or 5.0 mg.l-1 benzyladenine (BA) and 0.0, 0.1, 0.25, 0.5, or 1.0 mg 1-1 naphthalene acid (NAA) in factorial combination. Rooting medium consisted of basal medium supplemented with 0.001 mg 1-1 NAA dispensed into 55X70 mm jars (42.5 ml). The pH was adjusted to 5.7 to 5.8 with NaOH and HCL prior to autoclaving for 20 minutes at 121°C, 124 kPa.
Primary callus initiated on basal medium supplemented with either 0.5, 1.0, or 2.5 mg.l-1 BA was subcultured on basal medium supplemented with either the same level of BA or the next two higher levels of BA. Cultures from treatments producing less friable or morphogenic callus were then subcultured in the same manner for each new level of BA.
Cotyledon cultures were grown for 28 days either in the dark or under 16 hr photoperiods from cool white fluorescent lamps (~50 µEm-2s-1) at 25°C. Subcultured callus was transferred every 28 days. Cultures with shoots were transferred to basal medium with 0.1 mg.l-1 BA for shoot elongation. Shoots were excised from cotyledons or callus clumps and rooted under 16 hr photoperiods from cool white fluorescent lamps (50 µEm-2s-1). Rooted shoots were transferred to sterile 1:1 Jiffy mix:soil contained in Plant Cons (Flow Laboratories, McLean, VA 22102) When shoots and roots began active growth, plants were transferred to 6″ plastic azalea pots and acclimated in a greenhouse mist chamber for seven days before placement under in vivo conditions.
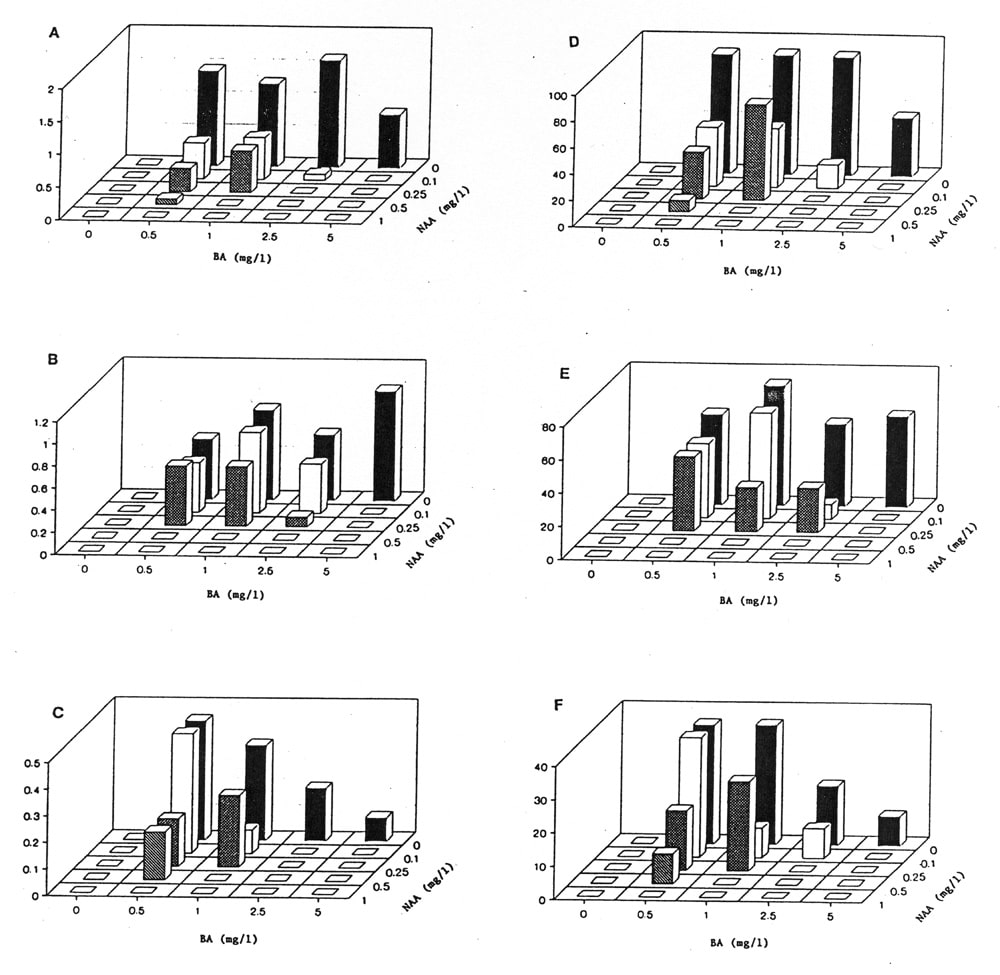
The effect of the absence or presence of light was similar to that previously reported on medium supplemented with kinetin and indoleacetic acid (IAA). Cotyledons cultured in the light formed green or white callus while those cultured in the dark formed friable white callus. However, unlike the kinetin-IAA medium there was direct regeneration of shoots from cotyledons on basal medium supplemented with 0.0, 0.1, or 0.25 mg.l-1 BA combined with 0.0, 0.1, or 0.25 mg.l-1 NAA, and 5.0 mg.l-1 BA combined with 0.0 mg.l-1 NAA (Fig. 1). In general shoot number decreased as NAA concentration increased. Media lacking BA formed progressively more roots with numerous root hairs as the NAA concentration increased. This pattern of root growth was the same for cotyledons grown both in the dark or the light.
All three cultivars developed shoots from subcultured callus when transferred as follows: 0.5–0.5–0.5 or 0.5–0.5–1.0 mg.l-1 BA. Other successful treatment combinations were as follows: ‘Hales Best’ and ‘Perlita’ 1.0–1.0–1.0 mg.l-1 BA. In general when callus was transferred to higher levels of BA friable nonmorphogenic callus overgrew the shiny green morphogenic callus previously formed.
We previously reported that ‘Hales Best’ had the highest morphogenic potential on basal medium supplemented with kinetin and IAA (2). On basal medium supplemented with BA and NAA, ‘Perlita’ had the highest morphogenic potential, followed by ‘Hales Best’ and ‘Iroquois’. Optimum BA and NAA levels varied with cultivar. For indirect regeneration, ‘Perlita’ also had the highest morphogenic response followed by ‘Hales Best’ and ‘Iroquois’.
Figure 1. A-C) Average rating of eleven light-grown cotyledons. Rating scale 0=no shoots, 0-1=1-10 shoots, 1-2=11-20 shoots. A) ‘Perlita’ B) ‘Hales Best’ C) ‘Iroquois’. D-F) Percentage of eleven light-grown cotyledons forming shoots. D) ‘Perlita’ E) ‘Hales Best’ F) ‘Iroquois’.
Literature Cited
- Halder, T. and V.N. Gadgil. 1982. Shoot bud differentiation in long-term callus cultures of Momordica and Cucumis. Ind. J. Exp. Biol. 20:780-782.
- Mackay, W.A., T.G. Ng and F. Hammerschlag. 1988. Plant regeneration from callus of Cucumis melo L. Cucurbit Genetics Coop. 11:33-34.
- Moreno, V., M. Garcia-Sogo, I. Granell, B. Garcia-Sogo, and L. A. Roig. 1985. Plant regeneration from calli of melon (Cucumis melo L., cv. Amarillo Oro). Plant Cell Tissue Organ Culture 5:139-146.
- Smith, S., K. Dunbar, R. Niedz, and H. Murakishi. 1988. Factors influencing shoot regeneration from cotyledonary explants of Cucumis melo. In Vitro 24:57A.