Cucurbit Genetics Cooperative Report 14:78-80 (article 29) 1991
G.S. Bokelmann, C.I. Jarl, and A.J. Kool
Zaadunie Research, Plant Biotechnology Div., P.O. Box 26, NL-1600 AA Enkuhuizen, The Netherlands
Introduction
Successful plant regeneration from explants of melon have been reported by a number of authors (for review see 2). Publications on plant regeneration from protoplasts still remain scarce and the genotypes used are limited (1, 3, 4, 5). In the publications by Debeaujon and Branchard (3) and by Moreno et al. (5), plants of ‘Cantaloupe charentais’-type were used as starting material, while Li et al. (4) used a chinese cultivar ‘Xinjang’. Here we present a method by which normal plants were obtained by organogenesis from protoplasts of different genotypes.
Materials and Methods
Nine different genotypes were tested in total, of which 4 commercial varieties and 5 parental lines. The cotyledons of 7-days-old-in vitro plants were used for isolation. The cotyledons were placed for a few days (2-3 d) in dark at room temperature, on a M/S medium supplemented with 1.0 mg/1 NAA and 1.0 mg/1 BAP, prior to isolation. Isolation was carried out overnight (16 h) at 28 ˚ C in an enzyme soilution (0.8% [w/v] Cellulase RS Onozuka, 0.4% [w/v] Macerozume R10 Onozuka, 0.1M glycine, and 0.4M mannitol in 1/4 strength MS salt solution). Isolated protoplasts were suspended at a density of 5 x 104 protoplasts/ml in a modified B5 medium consisting of B5 salts, KM8p vitamines, 0.25 M mannitol, 0.17 M glucose and 0.034 M sucrose [550 mosm], 0.2 mg./1 2,4-D, 1.0 mg/1 NAA and 0.5 mg/1 BAP. After 3 weeks, fresh culture medium without mannitol was added. At this time, Seaplaque agarose was added to give a final concentration of 0.2% [w/v]. After another 3-4 weeks, minicalli appeared and could be transferred to a shoot-initiation medium. For five of the cultivars, several different hormone combinations were tested. Four different cytokinins were tested at a range of concentrations: KIN (1.0-10.0 mg/l), BAP (0.2-5.0 mg/l), ZEA (0.1-5.0 mg/l and 2-IP (0.01-2.5 mg/l). For four other cultivars, an addition of 0.5 mg/l ZEA was used as standard. The shoot-initiation sphase took about 3 weeks, after which the calli with the induced shoot primordia were transferred to a medium for the development of shoots. Also in this case, different combinations of the following hormones were tested for five of the cultivars: IAA, NAA, BAP, ZEA, and GA3. For the other four tested cultivars, the combination of 0.1 mg/l IAA and 0.2 mg/l BAP was used. At this stage, developing shoot primordia could be dissected out for subculturing on the same medium. Also different gelling agents were used in different concentrations: 1.0% [w/v] of agar (Merck) , agarose 13.20 (Duchefa) and Microagar (Duchefa), while Gelrite (Duchefa) was testedin concentration of 0.2, 0.5, and 1.9% [w/v]. Finally, a medium supplemented with 0.03 mg/1 NAA was used for rooting.
Results and Discussion
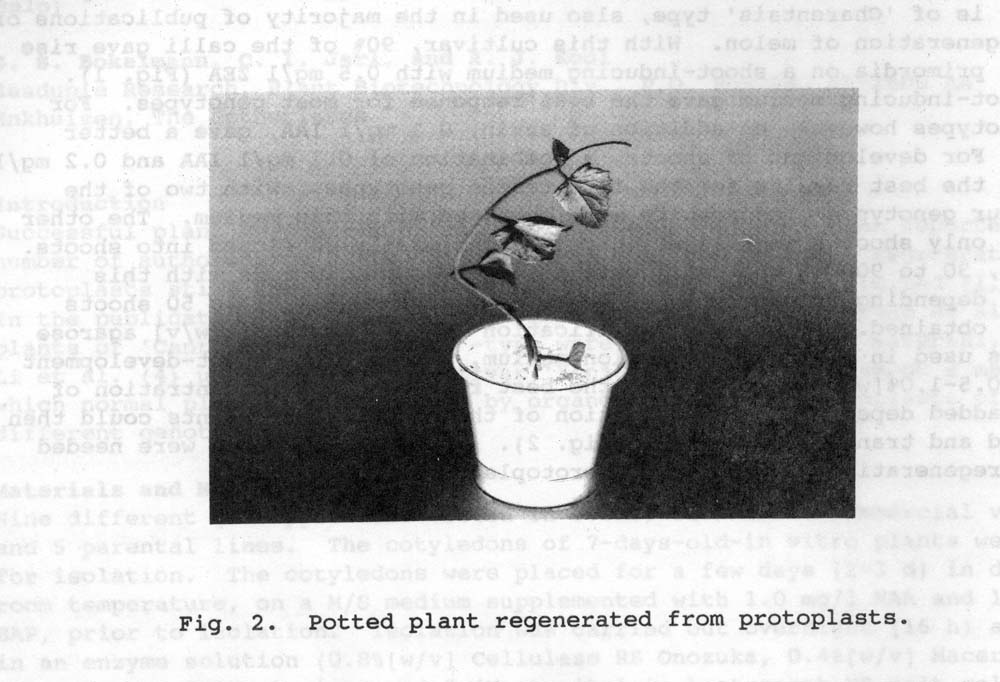
The highest plating efficiency was obtained when cotyledons were used as explant material. The preculture was also seen to increase the division frequency substantially. AFter one week in culture medium, the division frequency varied between 30 to 90% according to genotype. Our best responding cultivar is of “Charenbtais’ type, also used in the majority of publications of plant regeneration of melon. With this cultivar, 90% of the calli give rise to shoot primordia on a shoot-inducing medium with 0.5 mg/l ZEA (Fig. 1). This shoot-inducing medium gave the best response for most genotypes. With two of the other four genotypes good results were obtained with this medium. The other two gave only shoot primordia which only occasionally developed into shoots. In total, 30 to 90% of the calli gave rise to shoot primordia with this sequence depending on genotype. Fom each of those calli, 1 to 50 shoots could be obtained. To prevent vitrification of the plants, 1% [w/v} agarose 13.20 was used in the shoot-induction mediium, while in the shoot-development medium, 0.5-1.0% [w/v] Gelrite gave the best results. The concentration of Gelrite added depended on the condition of the plants. The plants could then be rooted and transferred to soil (Fig. 2). In total, 6 months were needed for the regeneration of plants from protoplasts.
Fig. 1. Shoot primordia obtained with out best cultivar, a ‘Cantaloupe charentais’ type.
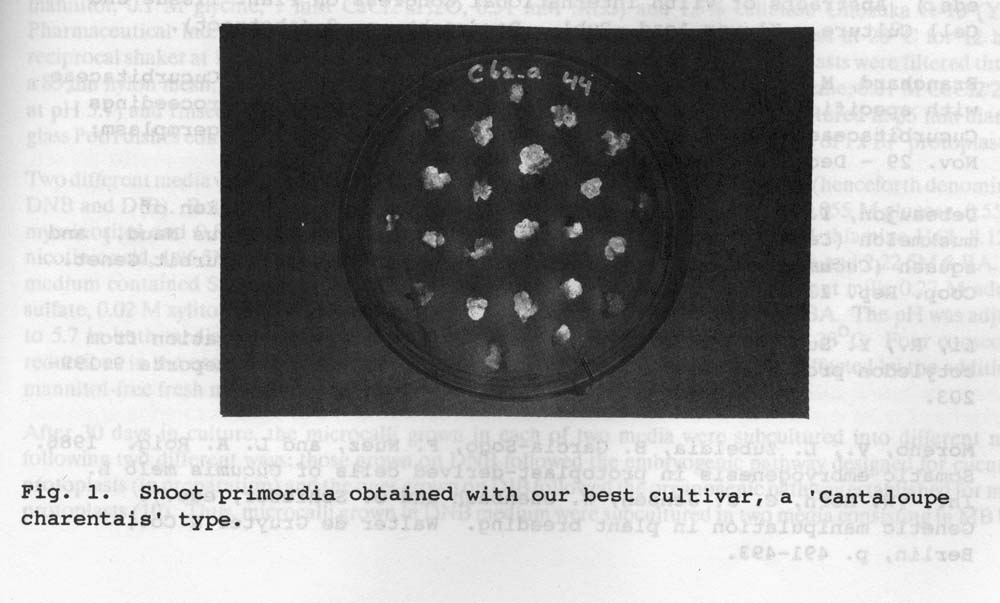
Fig. 2. Potted plant regenerated from protoplasts
Literature Cited
- Bokelmann, G.S., C.I. Jarl, and A.J. Kool. 1990. An efficient method for plant regeneration from protoplasts of commercial varieties of melon. In: (H.J.J. Nijkamp, L.H.W. van der Plas & J. Aartrijk, eds.) Abstracts of VIIth International Congress on Plant Tissue and Cell Culture. Kluwer Acad, Publ. Dordrecht, p.8 (abstract).
- Branchard M. and I. Debeaujon. Somatic embryogenesis in Cucurbitaceae with specific reference to muskmelon Cucumis melo L.). Proceedings Cucurbitaceae 89: Evaluation and enhancement of cucurbit germplasm; Nov, 29 – Dec. 2, 1989; Charleston, SC, USA: 9-20.
- Debeaujon, I. and M. Branchard. 1990. Somatic hybridization of muskmelon (Cucumis melo L.) with kiwano (Cucumis metuliferus Naud.) and squash (Cucumis pepo L.) by protoplast electrofusion. Cucurbit Genet. Coop. Rep. 13:36-39.
- Li, R. Y. Sun, Zhang, and X. Li. 1990. Plant regeneration from cotyledon protoplasts of Xinjiang muskmelon. Plant Cell Reports 9:199-203.
- Moreno, V., L. Zubeldia, B. Garcia-Sogo, F. Nuez, and L.A. Roig. 1986. Somatic embryogenesis in protoplast-derived cells of Cucumis melo L. In: (W. Horn, C.J. Jensen, W. Odenbach and O. Schieder, eds.). Genetic manipulation in plant breeding. Walter de Gruyter & Co., Berlin. p. 491-493.