Cucurbit Genetics Cooperative Report 16:61-63 (article 22) 1993
H.T. Lim, C.D. Boyer
Department of Horticulture, Kangwon National University, Chuncheon, 200-701, The Republic of Korea; Department of Horticulture, The Pennsylvania State University, University Park, PA 16802.
Introduction: Many agriculturally important processes take place in the various developmental states of plastids. Chloroplasts are extensively studied due to their photosynthetic process. Chromoplasts, the principal carotenoid-bearing organelles in higher plants, are the major sites of vitamin A precursors for fruits and vegetables. In most of higher plants, chloroplasts are much alike in morphology, but chromoplasts are found to be in a great diversity of shapes and sizes, especially during plastid differentiation.
The understanding of plastid differentiation and interconversion is of fundamental biological interest. We chose Cucurbita pepo, for they display a fascinating array of colors during fruit development. the biochemical aspects of carotenoid accumulation in the chromoplast of different squash varieties were studied by Schafer et al. (6). We reported restriction site and genetic maps of chloroplast DNA of Cucurbita pepo, which corresponds to 153 kb in size (3).
Changes in plastid ultrastructure from two genotypes affecting fruit pigment (YY B+B+ : fruits is initially green, then yellow) and YY BB: precociously yellow) of squash with isogenic backgrounds were compared at different stages of fruit development.
Materials and Methods. Fruits of two isogenic lines of squash (c.v. Early Prolific) were harvested at five developmental stages: 2 days postpollination, at pollination, 3 days postpollination, 10 days postpollination, and 20 days postpollination. We also used other squash varieties which display distinct green and yellow colors representing chloroplasts and chromoplasts, respectively. Small pieces of squash fruits were cut from the pericarp of fruits at five progressie stages, fixed in 3% glutaraldehyde in a 0.06 M phosphate buffer (pH 7.3) in ice for 3 hrs, and post-fixed it in 2% OsO4 for 2 hrs. The samples were dehydrated through a graded series of acetone and propylene oxide and then embedded in Epon resin. Thin sections were stained with 6% uranyl acetate for 90 min and lead citrate for 6 min (4) and observed with an electron microscope (Phillips) at 80 V.
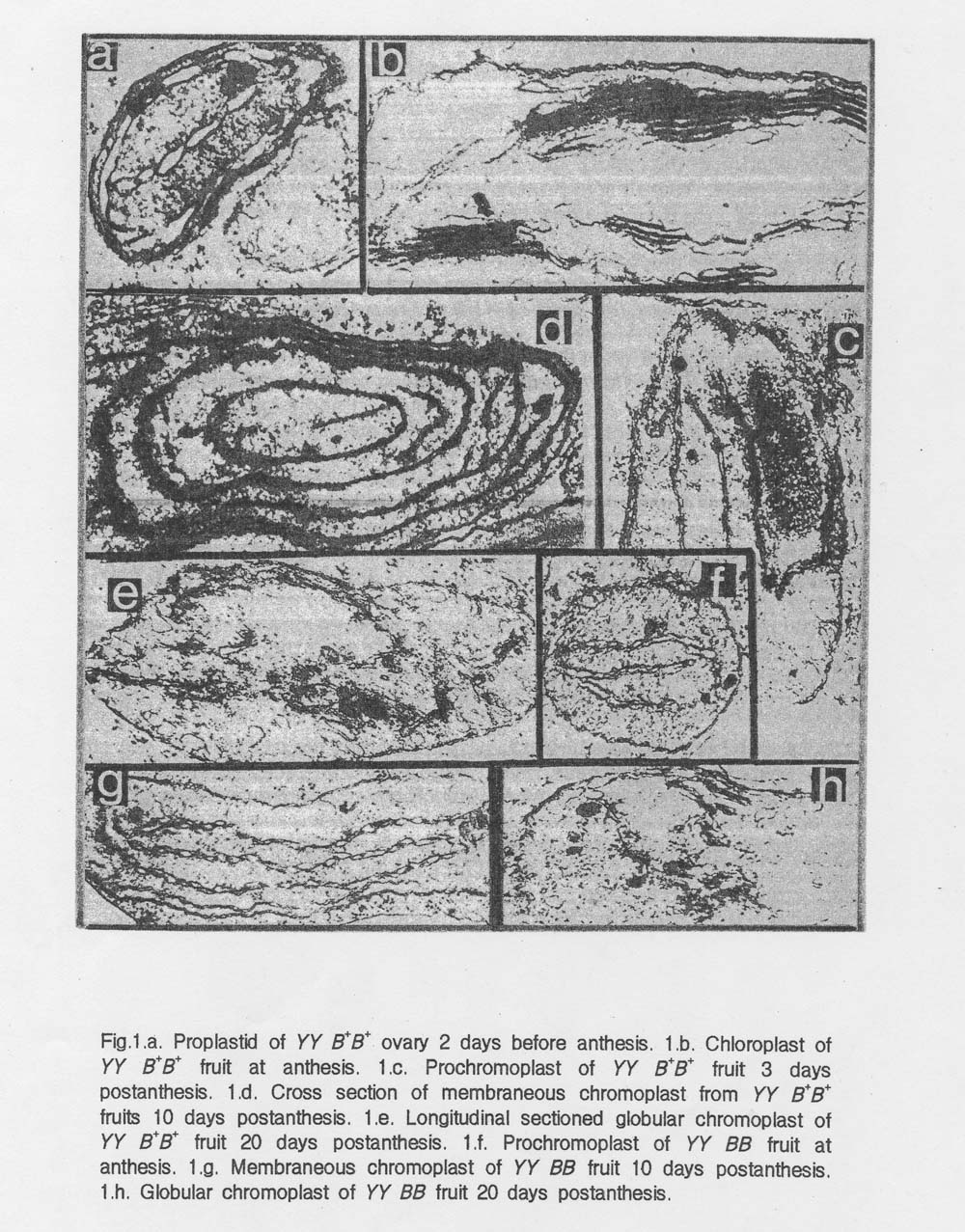
Results and Discussions: Longitudinal section of chloroplast of green zucchini fruits showed the granal and stomatal thylakoid network similar to that observed in leaf tissue of Early Prolific squash.
Appearance of plastoglobuli and internal membrane structure in prochromoplasts paralelled the disappearance of thylakoid materials in chloroplasts in YY B+B+ fruits. Therefore, we assume that thylakoid materials in chloroplasts may be used for the formation of internal membranes and plastoglobull during chromoplast differentiation (Fig. 1. a-e).
According to their structure and pigment composition of YY B+B+ fruits and of tomato fruits (2), we suggest that chromoplasts undergo structural changes from the membranous chromoplasts to the globular chromoplasts (Fig. 1. d-e).
Chromoplasts in fruits were indeed derived directly from proplastids in precocious (YY BB) fruits (Fig. 1.f-h). It was reported that chromoplasts of the precociously pigmented yellow portion of bicolor ornamental gourd were differentiated directly from proplastids (1).
Very similar line structure of plastids was observed in mature fruits of both genotypes. Based on previous hypothesis (6) and our results, we suggest that B and Y genes control the timing of chromoplast or chloroplast appearance rather than structure or content of plastids.
Fig. 1.a. Proplastid of YY B+B+ovary 2 days before anthesis. 1.b. Chloroplast of YY B+B+fruit at anthesis. 1.c. Prochromoplast from YY B+B+ fruit 3 days postanthesis. 1.d. Cross section of membraneous chromoplast from YY B+B+ fruits 10 days postanthesis. 1.e. Longitudinal sectioned globular chromoplast of YY B+B+ fruit 20 days postanthesis. 1.f. Prochromoplast of YY BB fruit at anthesis. 1.g. Membraneous chromoplast of YY BB fruit 10 days postanthesis. 1.h. Globular chromoplast of YY BB fruit 20 days postanthesis.
Literature Cited
- Devide, Z. 1970. Ultrastructurl changes of plastid in ripe fruits of Cucurbita pepo cv. ovofera. Acta Bot. Croat. 29:57-62.
- Harris, W.M. and A.R. Spurr. 1969 Chromoplasts of tomato fruits. 1. Ultrastructure of low-pigment and high-beta mutants. Carotene analyses. Amer J. Bot. 56 (4) : 369-379.
- Lim, H-T., I. Gounaris, R.C. Hardison, and C.D. Boyer. 1990/ Restriction site and Genetic Map of Cucurbita pepo chloroplast DNA. Chrrent Genetics. 18:273-275.
- Reynolds, E.S. 1963. The use of lead citrate at high pH as an electron opaque stain in electron microscopy. J. Cell Biol. 17: 208-212.
- Schaffer, A.A., C.D. Boyer, and T. Gianfagna. 1984. Genetic control of plastid carotenoids and transformation in the skin of Cucurbita pepo L. fruit. Theor. Appl. Genet. 68: 493-501.