Cucurbit Genetics Cooperative Report 19:32-33 (article 12) 1996
Michael J. Havey
USDA Agricultural Research Service and Dept. Horticulture, 1575 Linden Drive, University of Wisconsin, Madison, WI 53706
Introduction. Resistance in cucumber (Cucumis sativus L.) to Cucumber Mosaic Virus (CMV) traces back to an accession of ‘Tokyo Long Green’ and its inheritance has been characterized by numerous researchers (1,2,4). CMV resistance shows a dominance type expression, but is affected by background genotype, genetic modifiers, and environment. Provvidenti (3) discovered that the line ‘TMG-1’ is resistant to CMV, but did not report its inheritance. Herein, I report the initial characterization of the CMV resistance in TMG-1 and compare it to other known sources of resistance.
Materials and Methods. An isolate of CMV (522) was the gift of Dr. R. Provvidenti (Geneva, NY) and was maintained by monthly transfers on ‘Zucchini Select’ squash. Approximately 5 cm2 of freshly harvested expanding leaf tissue was ground in a mortar in 5 ml of phosphate buffer (0.05 M K2HP04, pH 8.8) until tissue was completely homogenized. Fully expanded cotyledons were dusted with carborundum and the virus suspension inoculated by gentle rubbing with the pestle. Plants were maintained in insect-proof cages. Sources of CMV resistance were ‘Wisconsin SMR18’, ‘Marketmore 76’ (MM76), and TMG-1. Single plants from SMR18, MM76, and TMG-1 were grown,propagated by cuttings, and the root stock inoculated with CMV as described above, ‘Straight 8′ plants (susceptible to CMV) were similarly propagated and the root stocks inoculated with CMV to identify a single susceptible plant (ST8-5), After determining the virus phenotype,rootstocks were discarded. Propagules of single CMV-resistant SMR18. MM76, and TMG-1 plants were crossed as males to propagules of ST8-5. Single F1 plants were self-pollinated to generate the F2 families. Single F2 families were chosen randomly and 75, 45, and 53 plants self-pollinated to generate F3 families from ST8-5, x SMR18, ST8-5 x MM76, and ST8-5 x TMG-1, respectively.
For each family, at least two replications of 10 plants were evaluated for CMV resistance. Cucumber plants were germinated in vermiculite for 5 days and single seedlings transplanted to 12.5- cm plastic pots with steamed compost:field-soil:peat:sand (1:1:1 on a volume basis) mixture. Ten to 14 days after transplanting, expanding first or second true leaves were dusted with carborundum and inoculated by three gentle strokes of the pestle over the leaf. Two weeks after the first inoculation, the newest approximately one-half expanded true leaf was dusted with carborundum and re-inoculated with the same virus. Two weeks later, the plants were scored as 1 = no symptoms to a slight mosaic on lower leaves; 3 = slight mosaic limited on lower leaves; 5 = slight mosaic on upper leaves’ 7 = mosaic on upper leaves, and 9 = severe mosaic on upper leaves.
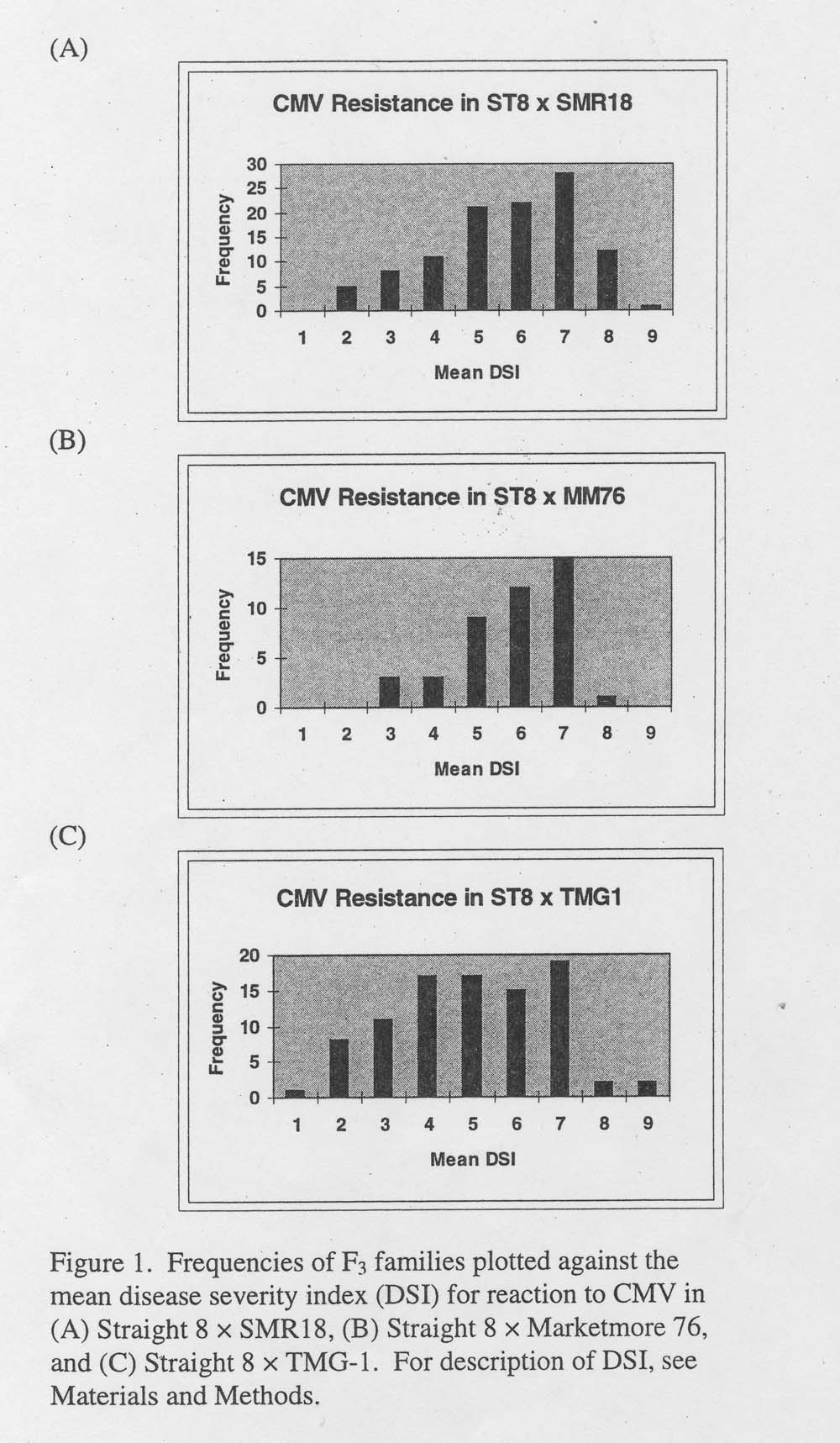
Results and Discussion. CMV resistance in SMR18 and MM 76 originates from ‘Tokyo Long Green’ (H. Munger, personal communication). Observations by cucumber breeders indicate that MM76 possesses a higher level of resistance to CMV than SMR18. For segregating families using SMR18 and MM76 as sources of CMV resistance, too many susceptible families were observed to allow adequate fit to the single dominant gene hypothesis proposed by Wasuwat and Walker (4) (Figure 1). TMG-1 appeared to be a better sources of resistance than SMR18 or MM76 and a greater frequency of resistant F3 families were observed (Figure 1). This is indirect evidence that TMG-1 may possess a CMV resistance different from either SMR18 or MM76. Because further characterization and tests of allelism are required, genotypes at putative CMV-resistance loci were not assigned.
Figure 1. Frequencies of F3 families plotted against the mean disease severity index (DSI) for reaction to CMV in (A) Straight 8 X SMR18, (B) Straight 8 X Marketmore 76, and (C) Straight 8 X TMG-1. For description of DSI, see Materials and Methods.
Literature Cited
- Cohen, S., E. Gertman and N. Kedar. 1971. Inheritance of resistance to melon mosaic virus in cucumber. Phytopathology 61:253-255.
- Kooistra, E. 1969. The inheritance of resistance in Cucumis virus 1 in cucumber. Euphytica 18:325-332.
- Provvidenti, R. 1985. Sources of resistance to viruses in two accessions of Cucumis sativus. Cucurbit Genet.Coop Rpt. 8:12.
- Wasuwat, S. and J. Walker.1961. Inheritance of resistance in cucumber to cucumber mosaic virus. Phytopathology 51:423-428.