Cucurbit Genetics Cooperative Report 6:32-34 (article 16) 1983
J. E. Staub, R. S. Kupper, and T. C. Wehner
USDA/ARS, Horticulture Department, University of Wisconsin, Madison, WI 53706 (first and second author); North Carolina State University, Raleigh, NC 27650 (third author)
Recently, several horticulturally important enzyme systems have come under investigation in the genus Cucumis (1, 2, 4). These systems and others have proven useful in studying the biochemistry (3, 5, 6, 7, 9, 10, 11, 12, 13, 14) of this genus and the taxonomy (8) of the Cucurbita. In addition, Robinson (11) has employed electrophoresis as an enzymatic tool for studying breeding material in his research program.
Limited information exists on linkage relationships and chromosomal mapping in Cucumis. It would be useful, therefore, to obtain reproducible and precise genetic markers which might lead to the characterization of linkage groups and eventually to the formation of a chromosome map of this horticulturally important genus.
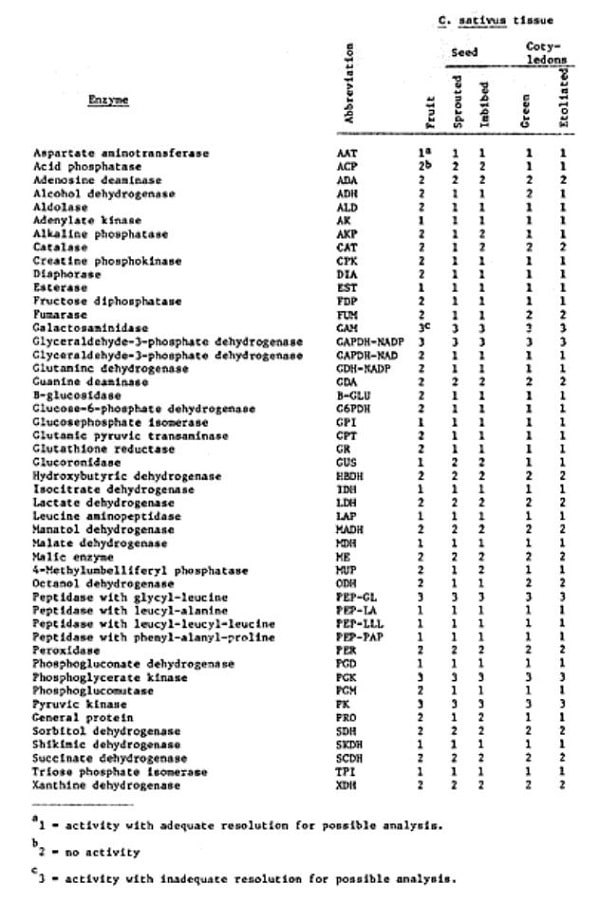
With this in mind, a study was initiated to: 1) survey the relative activity of 47 general metabolic enzymes and general protein in different tissues of selected Cucumis ssp., 2) determine which of these enzymes would give acceptable resolution for genetic analysis, and 3) determine the amount of enzyme polymorphism which exists in a broad based collection of Cucumis ssp.
Mature fruit, seed and cotyledons of two inbred C. sativus var. sativus lines (Gy2 and USDA 1379) and a C. sativus var. hardwickii (LJ 90430) representative were examined by horizontal starch gel electrophoresis using four buffer systems. Seeds were sampled as both imbibed (aerated in water at 32°C for 4 hrs) and sprouted (aerated in water at 32°C for 24 hrs; radicle = 5 mm), while cotyledons were harvested from two-week old seedlings grown in light (green) and in darkness (etiolated). The enzymes and general protein assayed (Table 1) showed differential activity within and between the tissues studied. There were no species differences observed with regard to specific activity within tissues but interspecific enzyme polymorphisms were evident. Enzymes were categorized as to their potential usefulness as genetic markers on the basis of their relative activity and resolution.
Green cotyledons were determined to be the tissue of choice for subsequent studies investigating the amount and types of available polymorphic loci. Sixty-four individuals (49 var. sativus, 7 var. hardwickii and 8 Cucumis species) were drawn from the Cucumis collection and were examined electrophoretically for 19 enzymes. Of these 19 enzymes, GPI, GR, MDH-NADP, PEP-PAP, PGD, and PGM were found to be polymorphic.
Studies are now underway to determine the genetic basis and inheritance of these polymorphic enzymes. When this is accomplished, identification of linkage groups and chromosome mapping of Cucumis may be possible.
Literature Cited
- Billett, E. E. and H. Smith. 1980. Control of phenylalanine ammonia-lyase and a cinnamic acid 4-hydroxylase in gherkin tissues. Phytochemistry 19:1035–1041.
- Gross, K. C. and D. M. Pharr. 1982. Cucumber fruit synthase isozymes. Phytochemistry 21:1241–1244.
- Gross, K. C., D. M. Pharr, and R. D. Locy. 1981. Growth of callus initiated from cucumber hypocotyls on galactose and galactose-containing oligosaccharides. Plant Science Letters 20:333–341.
- Klobus, G. 1980. Changes in phosphatase activity during germination and in early phases of cucumber seedlings growth. Acta Societatis Botanicorum Poloniae 49:91–103.
- McCreight, J. D., D. M. Pharr, R. L. Lower and H. N. Sox. 1976. Comparative study of ß-glucosidase from cotyledons and fruits of cucumber, Cucumis sativus. Physiol. Plant. 37:17–22.
- McFeeters, R. F., T. A. Bell and H. P. Fleming. 1980. An endo-polygalacturonase in cucumber fruit. J. Food Biochem. 4:1–16.
- Pharr, D. M., H. N. Sox, R. D. Locy and S. C. Huber. 1981. Partial characterization of the galactinol forming enzyme from leaves of Cucumis sativus L. Plant Science Letters 23:25–33.
- Puchalski, J. T. and R. W. Robinson. 1978. Comparative electrophoretic analysis of isozymes in Cucurbita species. Cucurbit Genetics. Coop. Rpt. 1:28.
- Rabinowitch, H. D. and D. Sklan. 1981. Superoxide dismutase activity in ripening cucumber and pepper fruit. Physiol. Plant. 52:380–384.
- Rice, C. A., K. S. Rymal, O. L. Chambliss and F. A. Johnson. 1981. Chromatographic and mass spectral analysis of cucurbitacins of three Cucumis sativus cultivars. J. Agric. Food Chem. 29:194–196.
- Robinson, R. W., J. T. Puchalski and A. C. de Ruiter. 1979. Isozyme analysis of the Megurk. Cucurbit Genetics Coop. Rpt. 2:17–18.
- Saltveit, M. E. and R. F. McFeeters. 1980. Polygalacturonase activity and ethylene synthesis during cucumber fruit development and maturation. Plant Physiol. 66:1019–1023.
- Smart, E. L. and D. M. Pharr. 1980. Characterization of a-galactosidase from cucumber leaves. Plant Physiol. 66:731–734.
- Wardale, D. A. and E. A. Lambert. 1980. Lipoxygenase from cucumber fruit: localization and properties. Phytochemistry 19:1013–1016.