Cucurbit Genetics Cooperative Report 8:71-73 (Article 27) 1985
Jaworski, A., P. M. Gorski*, S. Shannon, and R. W. Robinson
Department of Horticultural Science, New York State Agricultural Experiment Station, Geneva, NY 14456
Cucurbitacins (Cucs) are of concern to breeders due to their relation to insect resistance and fruit bitterness. When evaluating lines for Cucs, it is important that all samples are of the same plant part and of uniform age. Large differences in concentrations of Cucs were found in the placenta (locule contents, excluding seeds), flesh, and rind of fruit of Cucurbita equadorensis and the F1 hybrids of C. equadorensis x C. maxima and C. pepo x C. texana (Table 1) as well as C. texana (Table 2). In each genotype, the concentrations of Cucs was much higher in the placenta than in other parts of the fruit. Squash breeders using C. equadorensis as a source of virus resistance should be aware that, although the fruit flesh of this species is not bitter, the placenta contains toxic levels of Cucs; this is particularly of concern for summer squash derived from a cross with C. equadorensis, since the placenta is not often discarded as it is with winter squash.
Bitterness in the fruit of C. texana can be detected at anthesis by sampling placental tissue (Table 2). Cuc concentrations in placenta, flesh, and rind increased rapidly with age.
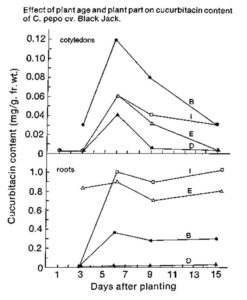
Concentrations of Cucs in the cotyledons and roots of C. pepo cv Blackjack changed rapidly during seedling development (Fig. 1). Because of these large differences in Cucs as a function of age and plant part sampled, it is important to specify the age, organ, and tissue sampled when reporting results.
We have used the HPLC method described by Ferguson et al. (1), with a modification in the method of extract purification. We use a single solvent (methanol:water, 45:55) to separate Cucs from most of the contaminants on silica plates with fluorescent indicator. Cucs are detected by quenching of fluorescence under 254 nm U.V. radiation (2).
| Cucurbitacins (µg/g fresh wt.)
|
||||||
| Genotype | Fruit part# |
E Glycoside |
D | I | B | E |
|
|
||||||
| C. equadorensis | P | tr | 500 | 60 | 140 | 30 |
| F | 0 | 10 | tr | 6 | tr | |
| R | 0 | 20 | tr | 20 | tr | |
| (C. maxima cv | P | tr | 390 | 50 | 350 | 60 |
| Buttercup x | F | O | tr | tr | tr | tr |
| C. equadorensis)F1 | R | O | 2 | 2 | 1 | 1 |
| C. pepo cv | P | tr | O | O | O | O |
| BlackJack | F | O | O | O | O | O |
| R | O | O | O | O | O | |
| (C. pepo cv | P | 290 | tr | 50 | O | tr |
| Blackjack x | F | 10 | O | tr | O | O |
| C. texana)F1 | R | 10 | O | tr | O | O |
|
|
||||||
| *P=placenta, F=flesh, R=rind. | ||||||
| Cucurbitacins (µg/g fresh wt.)
|
||||||
| Days after anthesis |
Fruit part# |
E Glycoside |
D | I | B | E |
|
|
||||||
| O | P | 364 | 0 | 161 | 200 | tr |
| F | 69 | 0 | 34 | 15 | tr | |
| R | tr | 0 | tr | tr | tr | |
| 4 | P | 454 | 0 | 46 | 52 | 198 |
| F | 32 | 0 | 52 | 47 | tr | |
| R | 13 | 0 | 6 | 11 | tr | |
| 14 | P | 1040 | 0 | 106 | 15 | 146 |
| F | 42 | 0 | 34 | tr | tr | |
| R | 13 | 0 | 3 | tr | tr | |
| 28 | P | 3560 | 0 | 284 | tr | 116 |
| F | 83 | 0 | 37 | tr | tr | |
| R | 38 | 0 | 1 | tr | tr | |
| 42 | P | 5920 | 0 | 2920 | 63 | 228 |
| F | 266 | 0 | 1480 | 4 | tr | |
| R | 48 | 0 | 1 | tr | tr | |
|
|
||||||
| #P=placenta, F=flesh, R=rind | ||||||
Literature Cited
- Ferguson, J. E., E. R. Metcalf and A. M. Rhodes. 1983. Influence of
cucurbitacin content in cotyledons of Cucurbitaceae cultivars upon
feeding behavior of Diabrotica beetles (Coleoptera:Chrysomelide).
Entom. Soc. Amer. 76:47-51. - Stoewsand, G. S., A. Jaworski, S. Shannon and R. W. Robinson. 1985.
Toxicologic response in mice fed Cucurbita fruit. J. Food Protection
48:50-51.
* On leave from Dept. of Biochemistry, Institute of Soil S