Cucurbit Genetics Cooperative Report 10:47-48 (article 27) 1987
D. Kenigsbuch and Y. Cohen
Department of Life Sciences, Bar-Ilan University, Ramat-Can 52100, Israel
Rosa (6) in his earliest experiments on the inheritance of flower type in muskmelon, showed that monoecism is dominant to andromonecism by a single pair of alleles. Poole and Grienball (5) proposed a two gene explanation of sex expression in muskmelon: AG is monoecious, Ag is gynomonoecious, aG is andromonoecious, and ag is hermaphrodite. These authors assumed that the gynoecious sex type in their F2 population (in the cross hermaphrodite x monoecious) was a transitory phenotype caused by environmental conditions and therefore considered it gynomonoecious. Rowe (7) suggested that gynoecious sex expression was controlled by modifying genes in addition to the major genotype A-gg. Peterson et al (4) reported on WI 998 muskmelon population in which stable, homozygous, gynoecious plants have been identified. This paper reports on the inheritance of gynoecism in the crosses gynoecious x monoecious and gynoecious x andromonoecious under greenhouse conditions.
Seeds of WI 998, obtained from C.E. Peterson, USDA, ARS, University of Madison, WI, were grown in the greenhouse. Plants were sprayed with AgNO3 (3) to induce the formation of hermaphrodite flowers, and then self-pollinated The 200 seeds collected from a single fruit produced all gynoecious plants in the field. A single plant was reproduced vegetatively in the greenhouse and used as a female or a male (following AgNO3 treatments) parent in reciprocal crosses with Cucumis melo PI 124111 (monoecious) or with the breeding line ’36’ (andromonoecious, homozygous, resistant to powdery and downy mildews derived from PI 124111 x Hemed x Perlita, see (1)).
F1 plants were all monoecious (Table 1) thus confirming earlier reports (2, 8). F2 populations segregated for sex type giving rise to 21 gynoecious (out of 293) plants (1/16) in the cross WI 998 x PI 124111, and 7 gynoecious (out of 312) plants (1/64) in the cross WI 998 x ’36’ (Table 1), indicating the possibility that gynoecism is recessive to monoecism by 2 genes, and recessive to andromonoecism by 3 genes. In the population derived from the BC WI 998 x (WI 998 x PI 124111) 1/4 of the plants were gynoecious. Gynoecious plants produced only pistilated flowers, until senescence.
Based on the data presented we propose that gynoecism in WI 998 is conferred by a recessive gene nn in addition to the major genotype A-gg. This gene is homozygous NN or heterozygous Nn in other sex types of muskmelon. The double recessive gene difference in the cross WI 998 x PI 124111 suggests A-G-N as monoecious. The triple recessive gene difference in the cross WI 998 x ’36’ suggests aa G-N- as andromonoecious. We assume that gynomonoecious is A-gg N and hermaphrodite is aagg N-. More data are currently collected to verify these assumptions. Gynoecious plants, resistant to powdery mildew were identified in the F2 population of the cross WI 998 x ’36’. This, and the fact that WI 998 carries Fom 3 for resistance against races 0 and 2 of Fusarium wilt (8) make it important for hybrid production.
Table 1. Frequency distribution of the F1 and the F2 populations in muskmelon from the crosses WI 998 x PI 124111, and WI 998 x ’36’, for sex expression.
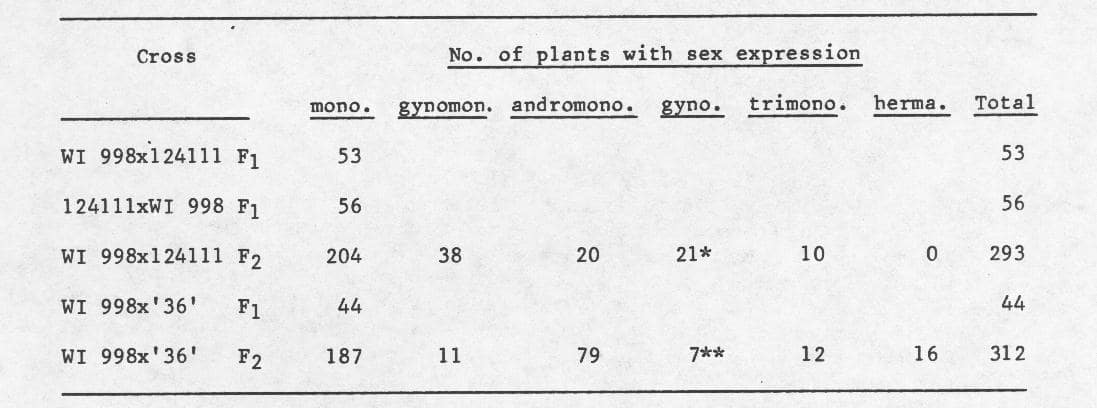
WI 998 – gynoecious; PI 124111 – monoecious; ’36’ – andromonoecious.
*21/293 = 1/16 with X2 = 0.422
**7/312 = 1/64 with X2 = 0.941
Literature Cited
- Cohen, Y., S. Cohen, and H. Eval. 1985. Inheritance of resistance to downy mildew in Cucumis melo PI 124111. Cucurbit Gen. Coop. Rpt. 8:36-38.
- Kubicki, B. 1969. Sex determination in muskmelon (Cucumis melo L.). Gen. Polon. 10:145-165.
- Owens, K.W., C.E. Peterson, and C.E. Tolla. 1980. Induction of perfect flowers on gynoecious muskmelon by silver nitrate and aminoethoxyvinylglycine. HortScience 15:654-655.
- Peterson, C.E., K.E. Owens, and P. R. Rowe. 1983. Wisconsin 998 muskmelon germplasm. HortScience 18:116.
- Poole, C.F., and P.C. Grimball. 1939. Inheritance of new sex forms in Cucumis melo L. J. Hered. 30:21-25.
- Rosa, J.T. 1928. The inheritance of flower types in Cucumis melo and Citrullus. Hilgardia 3:234-250.
- Rowe, P. R. 1969. The genetics of sex expression and fruit shape, staminate flower induction and F1 hybrid feasibility of a gynoecious muskmelon. Ph.D. Thesis, Michigan State University, 82 pp.
- Zink, F.W. and W.D. Gubler. 1986. Inheritance of resistance to races 0 and 2 of Fusarium oxysporum f. sp. melonis in gynoecious muskmelon. Plant Disease 70:676-678.