Cucurbit Genetics Cooperative Report 10:21-23 (article 14) 1987
J.E. Staub and M.J. Palmer
U.S. Department of Agriculture, Agricultural Research Service and Department of Horticulture, University of Wisconsin, Madison, WI 53706
Many important centers of origin are facing immediate threats to their native plant populations due to man’s encroachments. Resources are often inadequate to provide for all the expeditions needed to salvage critically needed germplasm from these areas and therefore efforts should be made to study existing collections. There are about 1131 documented accessions of the Cucumis species, excluding melo and sativus (1). Undoubtedly some of these are duplications. Nevertheless, given the available information, approximately 22, 17, 15, 11, and 12% of the Cucumis spp. accessions are held by the Philippines, German Democratic Republic, United States, Netherlands, and Japan, respectively. The reported African collections are held by Nigeria and South Africa and these are only 1 and 4% of the world collection, respectively. It becomes clear that countries which are in the major center of origin of these wild species do not retain large working collections. Therefore, research which will lead to a determination of the potential usefulness of these species must occur in countries which have relatively large collections, expertise, and adequate financial resources.
The existing collection of wild species houses resistances to several pests which are not currently found in C. sativus germplasm (eg. nematode, and green mottle mosaic). With the advent of workable recombinant DNA techniques, these resistances may in fact be transferred between cross -incompatible species. However, the accessions frequently lack information on essential characteristics which the plant breeder and/or molecular biologist will need for effective selection of initial germplasm. Resistance to economically important pathogens of commercial cucumber is of primary importance. Therefore, we felt it useful to obtain preliminary information on the susceptibility of 9 Cucumis spp. to downy mildew [Pseudoperonospora cubensis (Berk. & Curt.) Rostow.], and scab (spot rot) [Cladosporium cucumerinum Ellis & Arthur].
Materials. Seeds were sown in steam sterilized coarse grade vermiculite placed in wooden flats (52 x 36 x 7 cm.). Separate plantings were made for each pathogen. Resistance checks (GY-14 for downy mildew and ‘Wisconsin SMR- 18’ for scab) and susceptible checks (‘Wisconsin SMR-18’ for downy mildew and ‘Straight 8’ for scab) were seeded in the middle row of each flat. Plants which received no fertilization were grown in a greenhouse (22-35°C day, 20- 26°C night) under supplemental lighting (16 day) provided by Sylvania VHO fluorescent lamps at about 80 µmol s-1 m -2 . Plants were inoculated when cotyledons had expanded (approximately 7-10 days after sowing).
Since P. cubensis is an obligate parasite, it was maintained on infected ‘Wisconsin SMR-18’ seedlings placed in a 16°C growth chamber. Two to three weeks after inoculation, sporulation was induced by incubating the ‘SMR-18’ at 100% RH, 20°C for 24 hr. Cotyledons were placed in distilled water and rubbed with a finger to dislodge the sporangia and the concentration adjusted to 1. 2 x 10 5 sporangia/ml. Inoculum was maintained at 20°C for 1-2 hr. to induce zoospores. A 0.01-0.03 ml.. droplet of inoculum was placed on the center of the adaxial surface of one cotyledon using a Pasteur pipet.
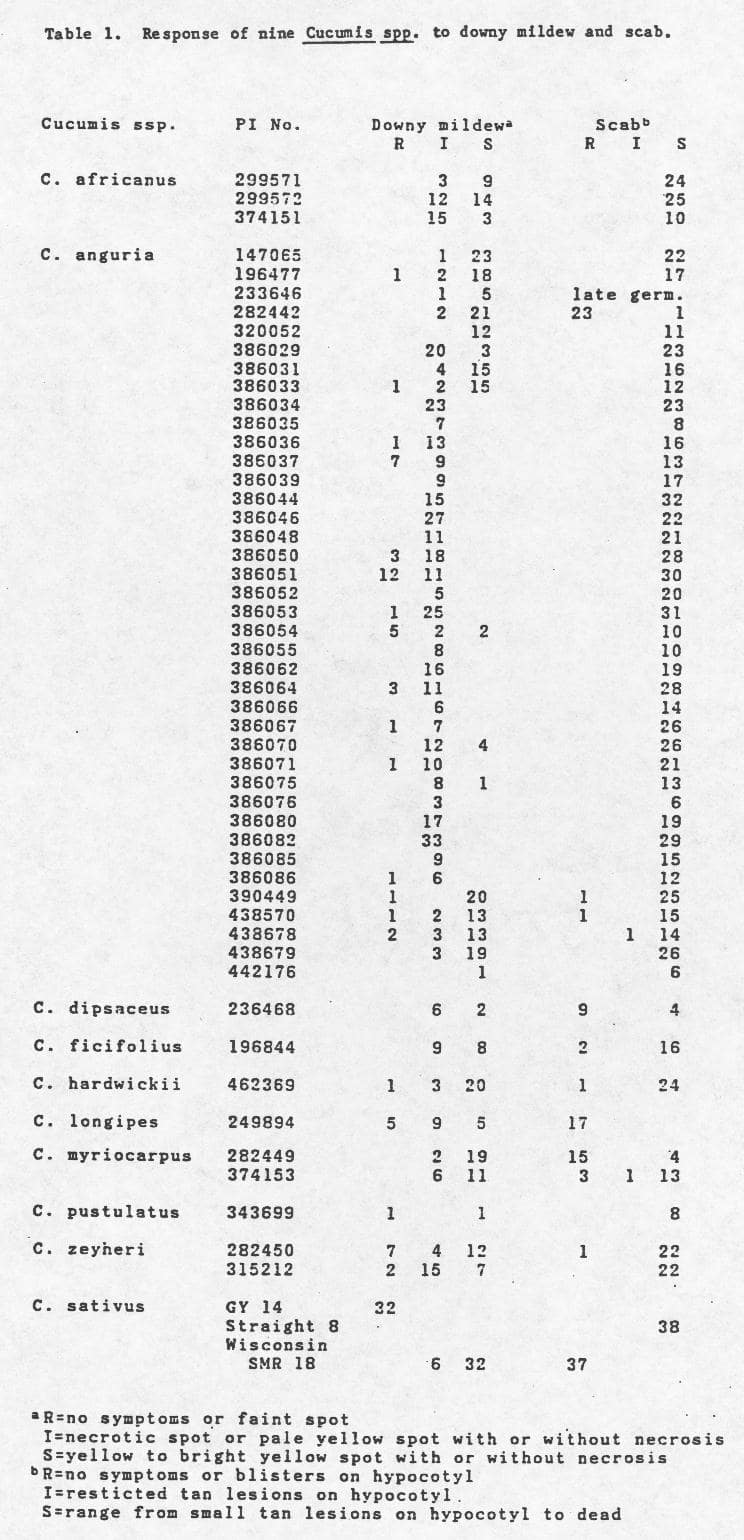
Seedlings were incubated in the dark at 100% RH, 20°C for 48 hr., and returned to the greenhouse. Seven to eight days after inoculation plants were rated as resistant (R), intermediate (I), or susceptible (S) (Table 1).
Three to six days before inoculation C. cucumerinum spores were streaked onto potato dextrose agar slants and incubated at 20°C. Inoculum was prepared by scraping the spores into distilled water and adjusting the concentration to 4 x 10 5 spores/ml. The inoculum was sprayed on the hypocotyl using a Badger (TM) air brush model 100XF. Plants were incubated in the dark at 100% RH, 20°C for 48 hr., and then were maintained in a greenhouse at 20°C. Six to nine days after inoculation plants were rated as resistant (R), intermediate (I), or susceptible (S) (Table 1).
Results. Of the 3 C. africanus accessions surveyed, all possessed some individuals which showed an intermediate (I) response to downy mildew, but were susceptible to scab. The C. anguria accessions PIs 386037 and 386051 possessed a relatively high frequency of individuals resistant to downy mildew. However, recent data (2,3) suggests that these accessions from Iran have been misclassified and are in reality C. melo. Only the PI’s 196477, 438570, and 438678 have resistance to downy mildew. Likewise, only PI 282442 provides resistance to scab. Collections of C. dipsaceus, C. ficifolius, and C. myriocarpus possesses individuals with intermediate resistance to downy mildew and resistance to scab. The C. hardwickii, C. longipes, and C. zeyheri had resistance to both pathogens. Although the germination of C. pustulatus was low, an individual was observed to be resistant to downy mildew. These data indicate that inter- and intraaccession variation for these diseases exist in the wild species studied. Using this type of information, selected Cucumis spp. with disease resistance can be used for transfer of other genes into commercial C. sativus germplasm.
Table 1. Response of nine Cucumis Spp. to downy mildew and scab.
Literature Cited
- Esquinas-Alcazar, J.T., and P.J. Gulick. 1983. Genetic resources of cucurbitaceae. International Board for Plant Genetic Resources. AGPG: IBPGR 48:83.
- Leeuwen, L. Van, and A.P.M. den Nijs. 1980. Problems with identification of Cucumis L. taxa. Cucurbit Genet. Coop. Rpt. 3:55-56.
- Staub, J., L. Fredrick, and T.L. Marty. Electrophoretic variation in cross -compatible wild diploid species of Cucumis. Can. J. Bot. (In Press).