Cucurbit Genetics Cooperative Report 10:18-20 (article 13) 1987
Staub, J.E. and L. Crubaugh
U.S. Department of Agriculture, Agricultural Research Service and Department of Horticulture, University of Wisconsin, Madison, WI 53706
Cucumber (Cucumis sativus L.) plants under stress. produce more staminate flowers than those grown under optimal conditions (1,2,4,8). For once-over mechanical harvest, processing cucumbers with uniform flowering and concentrated fruit set are essential (5). Among the genetic manipulations which has shown potential for increasing sex stability and uniform flowering is the chracterization and introduction of the hermaphroditic character (3). Hybrids made using hermaphrodites have been hypothesized to be sexually more stable under conditions of environmental stress (7).
Recently improved pollen induction on gynoecious inbreds achieved by using foliar applications of silver nitrate (9) or aminoethoxyvinylglycine (6)
instead of gibberellic acid, has resulted in increased interest in using gynoecious (G) x (G) hybrids. If hermaphroditic (H) pollen parents could be developed which conferred adequate yield and quality, then a less-costly pollen supply could be used in hybrid seed production. This and the fact that G x H hybrids may be sexually more stable makes this an attractive methodology.
However, a consistent, rapid test for gynoecious sex stability has not been developed. This has made identification of sex stable genotypes difficult, and has been at least partially responsible for the lack of widespread acceptance of the hermaphroditic character in plant breeding programs. Determining whether silver thiosulfate could be used to identify plants which possessed a more stable gynoecious character is thought to be desirable. If a dosage could be identified which would provide a consistent stress threshold, then rapid procedures might be developed for seedling screening for sex stability. Naturally a prerequisite for such a procedure would necessitate that the action of any chemical be unaffected by minor changes in the test environment (photoperiod, temperature, relative humidity, etc.).
A series of experiments were designed to determine at what threshold concentration and dosage silver thiosulfate would cause gynoecious genotypes to produce staminate flowers. In the first experimental sequence, a dosage of either 10 or 20 µl silver thiosulfate was administered at concentrations of 6, 3, 1.5, and 0.75 mM to cotyledons of 7-day-old seedlings of the gynoecious processing inbred line WI 1701 which had been germinated in pots 10 cm in diameter. Treatments were arranged in a randomized complete block design with 4 replications having 2 subsamples. Plants were grown to 10 flowering nodes in a standard growing medium (2 soil: 1 sand: 1 peat: 1 perlite by volume) in a greenhouse under a 16 hr. photoperiod at 25 to 28°C and 40 to 60% RH. The average quanta per day from solar radiation over 16 hrs. during this experimental period ranged from 423 to 843 µmol s-1 m-2 and plants were supplemented by fluorescent lights (Sylvania – F96Tl2/CW/VHO) providing approximately 40 µmol s-1 m-2 at the shoot apices to extend the photoperiod. Number of nodes with staminate flowers were recorded. Based on these data, potentially effective concentrations were isolated and used in 2 subsequent experiments where plants of WI 1701 were grown under similar environmental conditions as those described above, except that the average solar radiation ranged from 240 to 281 µmol s-1 m-2.
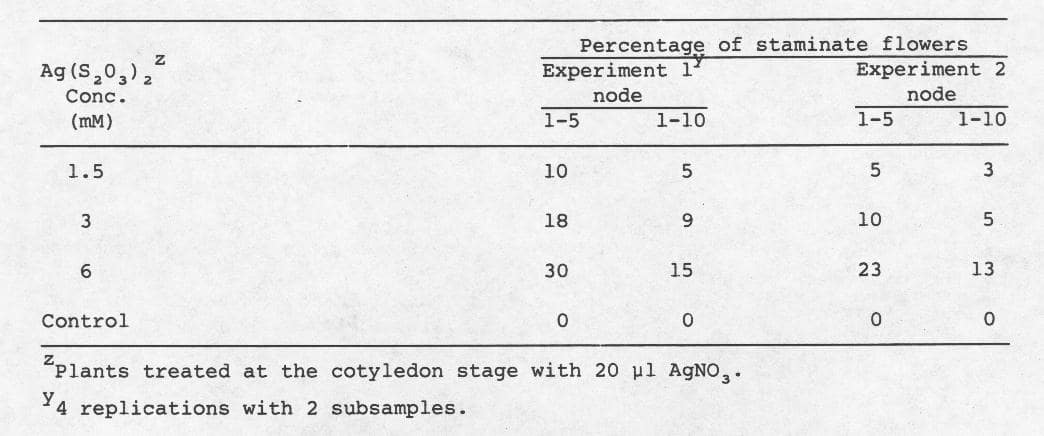
Data suggest that 20 µl was a consistent, effective dosage for the conversion of nodes to the staminate condition, except at 6mM (Table 1). The 20 µl dosage at 6 mM. was apparently above the threshold concentration (Table 1, exp. 1), but elicited a response in a second series of experiments (Table 2). Moreover, in the second series of experiments 1.5 mM at 20 µl was not as effective as 6 mM. The lowest effective concentration was 0.75 mM. (Table 1, exp. 2).
One explanation which might account for disparities observed between experiments was the difference in average quanta of solar radiation received by the plants during experimentation. If this hypothesis is correct, then effective threshold silver thiosulfate concentrations may vary and implementation of this procedure for sex stability testing will require careful control of environmental conditions.
Table 1. Mean percentage of staminate flowers after treatment of the USDA processing cucumber inbred line WI 1701 with Ag(S2O3)2 at the cotyledon stage.
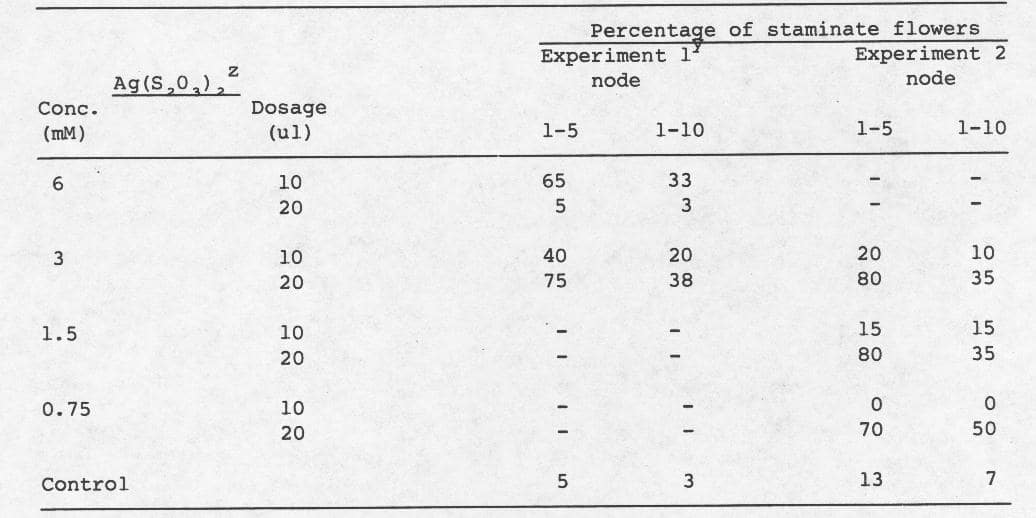
z Plants treated at the cotyledons stage; approximately 7 days after sowing.
y 4 replications.
Table 2. Mean percentage of staminate flowers after treatment of the USDA processing cucumber inbred line WI 1701 with Ag(S2O3)2at the cotyledon stage.
Literature Cited
- Cantliffe, D.J. 1981. Alteration of sex expression in cucumber due to changes in temperature, light intensity, and photoperiod. J. Amer. Soc. Hort. Sci. 106:133-136.
- Edmond, J. 1930. Season variation in sex expression of certain cucumber varieties. Proc. Amer. Soc. Hort. Sci. 27:329-332.
- Kubicki, B. 1965. New possibilities of applying different sex types in breeding. Genet. Pol. 6:241-249.
- Lower, R.L., O.S. Smith, and A. Ghaderi. 1983. Effects of plant density, arrangement, and genotype on stability of sex expression in cucumber. HortScience 18:737-738.
- Miller, C.H. and G.R. Hughes. 1969. Harvest indices for pickling cucumber in a once-over harvested system. J. Amer. Soc. Hort. Sci. 94:485-487.
- Owens, K.W., G.E. Tolla, and C.E. Peterson. 1980. Induction of staminate flowers on gynoecious cucumber by aminoethoxyvinylglycine. HortScience 15:256-257
- Pike, L.M. and W.A. Mulkey. 1971. Use of hermaphrodite cucumber lines in the development of gynoecious hybrids. HortScience 6:339-340.
- Tiedjens, V.A. 1926. Sex ratios in cucumber flowers as affected by different conditions of soil and light. J. Agr. Res. 36:721-746.
- Tolla, G.E. and C.E. Peterson. 1979. Comparison of GA 4/17 and silver nitrate for the induction of staminate flowers in a gynoecious cucumber line. HortScience 14:542-544.