Cucurbit Genetics Cooperative Report 11:29-32 (article 14) 1988
Jack E. Staub
U.S.D.A. / A.R.S., Department of Horticulture, University of Wisconsin, Madison, WI 53706
Improvement of cucumber (Cucumis sativus var. sativus L.; hereafter referred to as sativus) for low temperature germination and emergence ability has been the focus of several research programs in the U.S.A. (1, 3. 4). In contrast, screening and selection of cultivars for resistance to chilling injury after emergence has not been reported in the U.S.A. However, selection for growth of slicing cucumbers under conditions of cool temperatures (20˚C day and 15˚C night) and low light has been successful in Europe (2).
C. sativus var. hardwickii (R.) Alef. (hereafter referred to as hardwickii) is a progenitor or wild relative of sativus and was originally collected in the foothills of the Himalaya mountains. It is a potential source for increased yield in sativus. Because of its origin, hardwickii may be a good source for chilling resistance. Therefore, three experiments were designed in order to measure: 1) The effects of leaf handling on the measurement of photosynthesis rate and somatal conductance and; 2) The ability of hardwickii and sativus to recover from exposure to chilling.
Experiment 1. Sativus (WI 1606; gynoecious) and hardwickii (PI 215589; monoecious) plants were grown in 100-mm-diameter pots containing Promix in two controlled environment chambers at 30˚C under a 16 hour photoperiod (at 300 μmol ∙ s-1· m-2) in a randomized complete block design with 3 replications. At the 6-leaf stage, plants in both chambers were subjected to 5˚C for 24 hours (at 300 μmol ∙ s-1∙ m-2) and returned to 30˚C. The photosynthesis rate (PR) and stomatal conductance (SC) of the 2nd through 4th leaves (leaf 0 was the cotyledon) of plants in one chamber were measured using a portable photosynthesis system (LI-6000 Li-Cor, Inc., Lincoln, Nebraska) 2, 3, and 6 days after exposure. Plants in the second chamber were measured on day 6 after exposure. Analyses of variances were performed on measurements taken on day 6 after exposure.
Experiment 2. Sativus and hardwickii plants were grown as described in experiment 1. At the 6 leaf stage, the PR and SC of plants in one chamber was measured as previously described and then plants were exposed to 5˚C for 24 hours, returned to 30˚C and measured again after 2 days. In a second chamber, plants were measured only once 2 days after chilling exposure. Analysis of variance was performed on measurements taken 2 days after exposure.
Experiment 3. Sativus and hardwickii plants were grown as previously described. At the 6-leaf stage, plants in one chamber were exposed to 5˚C for 24 hours, returned to 30˚C, and measured for PR and SC 6 days after chilling as described above. Plants in a second chamber were grown continuously at 30˚C, and PR and SC measurements were taken immediately following those which had received chilling exposure. Plants were harvested and measured for leaf area, stem and leaf dry weight, and numbers of lateral branches and leaves. Analysis of variance was performed on PH and SC measurements taken 6 days after exposure and on morphometric characters.
Experiment 1 assessed the effect of handling and measurement of plants after exposure to 5˚C. No significant difference in PR and SC could be detected among plants measured several times and those measured only once suggesting that handling does not affect subsequent measurements of these plants (Figure 1). Significant differences in PR were recorded between sativus and hardwickii, but not for SC. Regardless of treatment, the sativus inbred always had a higher PR when compared to hardwickii. however, only when plants were measured 3 times did the sativus inbred have higher SC rates.
Experiment 2 assessed the effect of handling and measurement before and after chilling. Although there was no significant difference in the PR and SC rates of plants due to handling and measurement, the PR and SC rates of the sativus inbred was always higher than hardwickii (Figure 1). A closer inspection of the reaction of individual leaves suggested that when plants were measured before chilling, the results were inconsistent. At some leaf positions this response was elicited, while in others it was not. These inconsistent responses among leaves were not observed when plants were measured only once.
Experiments 1 and 2 indicted that handling and measurement before or after chilling exposure did not significantly affect PR or SC and, therefore, the interpretation of effects for these response variables. Experiment 3 indicate that, although chilling significantly lowers PH and increases SC (Figure 2), sativus consistently had higher PR and SC rates when compared to hardwickii regardless of treatment. A reduction in leaf area (54%), stem dry weight (52%), leaf dry weight (61%), lateral branch number (76%), and leaf number (59%) was recorded in plants exposed to chilling temperatures (data not shown).
The fact that hardwickii stomates are apparently open to a lesser degree ()lower SC rates) when compared to sativus may provide an explanation to the increased chilling injury of hardwickii. An hypothesis that explains the data is that hardwickii and sativus transpiration/photosynthesis ratios may be relatively equal, and stomatal response to low temperatures may be slower in hardwickii than in sativus making it more sensitive to chilling and affecting the recovery time.
Figure 1. Response of photosythetic rate and stomatal conductance in Cucumis sativus var. sativus L. (WI 1606) and Var. hardwickii (R.) Alef. (PI 215589) to 5°C in two experiments. Experiment 1 assesses the effect of handling and measurements of plants after chilling period. Experiment 2 compared plants grown under continuous 30°C to those measured before and after exposure to 5°C for 24 hours and then returned to 30°C.
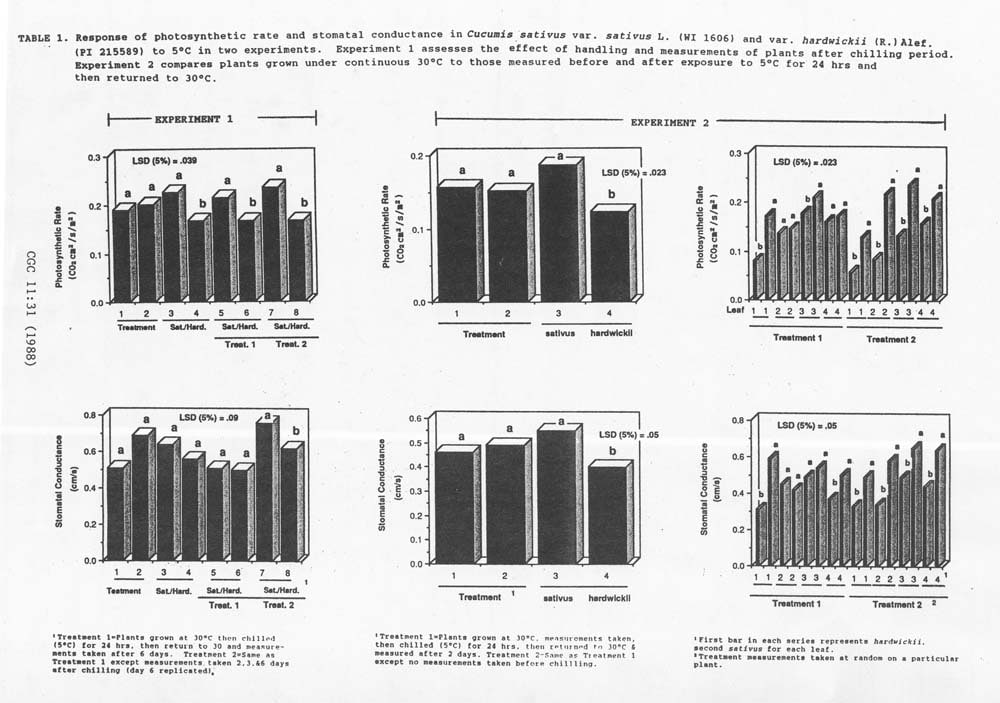
Figure 2. Response of photosynthetic rate and stomatal conductance in Cucumis sativus var. sativus L. (WI 1606P and var. hardwickii (PI 215589) to chilling (5°C) in Experiment 3.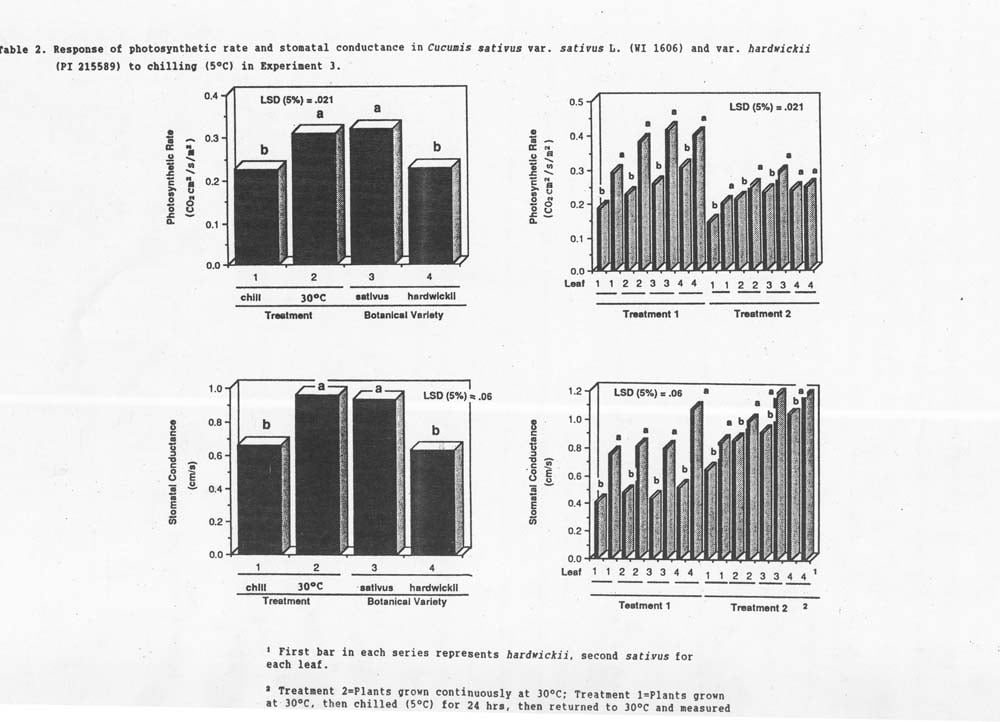
Literature Cited
- Lower, R.L. 1975. Measurement and selection for cold tolerance in cucumber. Pickle Pak Science. 4:8-11.
- Nijs, A.P.M. den. 1979. Low temperature adapted slicing cucumbers released. Cucurbit Genet. Coop. Rpt. 2:13.
- Staub, J.E., J. Neinhuis, and R.L. Lower. 1986. Effects of seed preconditioning treatments on emergence of cucumber populations. HortScience 21:1356-1359.
- Wehner, T.C. 1984. Estimates of heritabilities and variance components for low temperature germination ability in cucumber. J. Amer. Soc. Hort. Sci. 109:664-667.