Cucurbit Genetics Cooperative Report 12:29-34 (article 13) 1989
F.A. Tang and Z. K. Punja
Campbell Institute for Research and Technology, Campbell Soup Company, Route 1, Box 1314, Davis, CA 95616
The introduction of disease resistance into pickling cucumber (Cucumis sativus L.) is an essential component of all cultivar development programs. Efforts continue to identify new sources of resistance to major disease problems, such as root-knot nematodes (Meloidogyne incognita) and to viruses, such as zucchini yellow mosaic (ZYMV) and watermelon mosaic (WMV). The wild African horned cucumber (Cucumis metuliferus) PI 292190 has been shown to carry resistance to M. incognita and to ZYMV and WMV-1 (7, 15, 16) but not to WMV-2 (15). Efforts to introgress this germplasm by conventional sexual crosses have not yielded any success due to severe incompatibility barriers (3, 10). Somatic hybridization by protoplast fusion has been one approach that investigators have shown to be successful in transferring traits of interest from distantly related species to cultivated species in Brassica (14), Daucus (5), Lycopersicon (13), Nicotiana (4) and Solanum (1, 2, 6, 17). In this report, we describe results from studies aimed at establishing a procedure for the isolation and fusion of mesophyll protoplasts of two species, C. sativus and C. metuliferus. The ultimate goal is to identify somatic hybrids which may bring in traits of interest fromC. metuliferus into a C. sativus background.
Plant materials. Seeds of C. sativus Gy 14 and C. metuliferus PI 292190 were dipped into 70% ethanol for 20 sec. followed by a 20 min soak in a 20% solution of commercial bleach (Clorox, 5.25% sodium hypochlorite) to sterilize them, followed by 3 rinses in sterile, distilled water. The seed coats were excised under sterile conditions and the embryos transferred to Magenta boxes containing 50 ml of hormone-free Murashige and Skoog (12) basal medium (MS) with full strength macroelements and microelements, myo-inositol (100 mg/l), thiamine HCL (0.8 mg/l), 3% sucrose, and 0.65% Phytoagar. The pH of the medium was adjusted to 5.8 with 1 N KOH prior to autoclaving at 15 psi for 16 min. Boxes were incubated in a walk-in growth chamber set at 18/24°C night/day temperature regime with 16 hr/day photoperiod provided by cool-white fluorescent lamps (intensity of 160 mEM-2 sec-1). These in vitro cultures were used to provide plant materials for protoplast isolation. The first to third leaf from 15 to 21 day-old seedlings were used as the source of mesophyll protoplasts.
Isolation and purification of protoplasts. True leaves (1 g) were cut into 1-2 mm wide X 10 mm long strips with a scalpel under sterile conditions and placed in 20 ml of the enzyme solution in a 100 X 25 mm petri dish. The optimal concentration of pectinase (Sigma) and cellulysin (Calbiochem) required for both Gy 14 and for C. metuliferus was 0.5% and 1.0%, respectively. These resulted in yields of 5 to 6×106/g tissue. Enzyme solutions were prepared in modified MS medium containing half-strength major salts, full complement of minor salts and vitamins, 2% sucrose and 0.25M mannitol. The enzyme solution was sterilized by filtration using a syringe (B-D disposable) and Nalgene disposable filter unit (0.22 mM pore size). Tissues were incubated overnight (15 to 16 h) in the dark at 24±2°C on a reciprocating shaker set at 60 rpm. The resulting suspensions was passed through multilayers of sieve cloth (pore sizes from 50 to 300 mM to separate protoplasts from undigested plant debris. Two rinses in basal medium containing mannitol and centrifugation at 1200 rpm were conducted to remove the enzyme solution and purify the protoplasts. Protoplasts were concentrated as a dark green band at the meniscus of the Babcock bottles following purification. The pellet was removed with a Pasteur pipette and resuspended in basal medium and protoplasts were diluted to the desired density (2.5 to 3.0 x 104/ml) for fusion.
Protoplast fusion. One-half ml of the protoplast suspension of each species was mixed in a 60 X 15 mm petri dish and 1 ml of the following fusion treatments were tested: PEG M.W. 8,000 (8) at a concentration of 15% (in final volume), with or without 1% DMSO, for 20 min; high pH/Ca for 15 to 20 min (solution comprised of mannitol, 80 g/l; CaCl2.2H20, 7.35 g/l; glycine, 3.75 g/l; pH 10.0) (9). Following the treatments, the protoplast suspension was washed 2 to 3 times with basal medium to remove the fusigenic agents, and protoplasts were concentrated to a density of 2.5 to 3.0×104/ml.
Protoplast culture. Protoplasts of C. sativus at a density of 2/5 to 3.0×104/ml or at 0.5 to 0.6×104/ml, C. metuliferus protoplasts alone, and a mixture of the two species, were plated without any fusion treatment, and following the treatments described above, in MS medium with half strength major salts, full complement of minor salts and vitamins, 2% sucrose, and 0.25 M mannitol. Hormonal requirements were provided by 2,4-D/BA at 5.0/5.0 mM or NAA/BA at 5.0/2.5 mM. The suspension (2 ml) was added to soft agarose (0.4%) in 35 X 10 mm petri dishes. All dishes were incubated in the dark at 24 to 26°C in a growth chamber for the first 7 days, and then transferred to a 16 h photoperiod provided by cool-white fluorescent lamps, intensity of 100 mEM-2 sec-1. The days to first division, second-third division, and formation of minicalli were assessed for each species and fusion treatment. An additional treatment was imposed on these platings, namely that of a nurse culture (11). This was achieved by placing droplets of the protoplast suspension (with or without fusion treatments) in the center of the petri dish and placing along the periphery of the dish a 1.0 to 1.5 cm zone of mesophyll protoplasts of C. sativus at a density of 2.5 to 3.0 x 104ml, avoiding contact with the protoplasts under experimentation by providing a cell free circular zone about 1.5 cm wide.
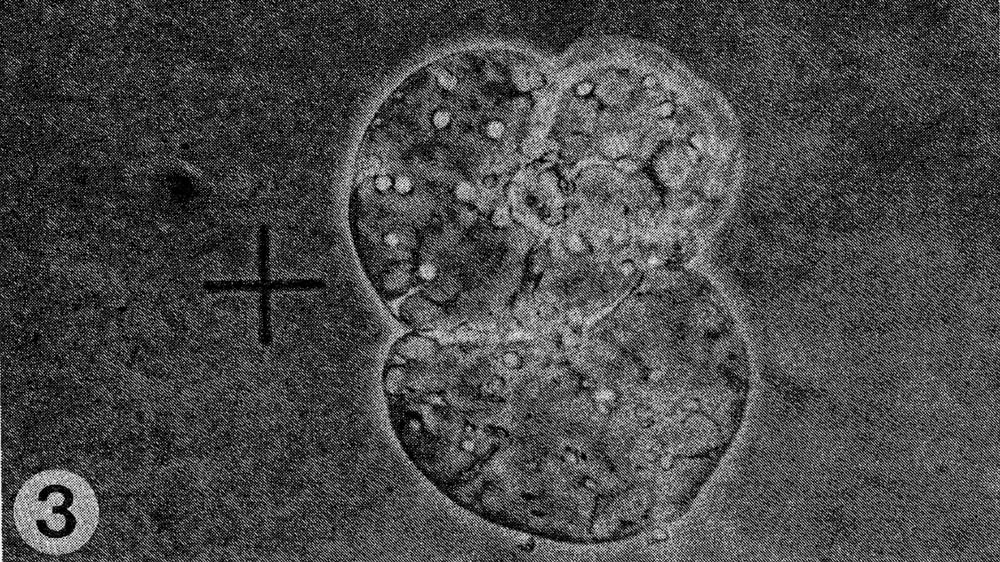
Results. The protoplast isolation procedure described gave high yields of good quality protoplasts of both species. Without imposing any fusion treatment, protoplasts of C. sativus formed minicalli within 13 days when plated at a density of 2.5 to 3.0 X 104/ml (Table 1). At a lower density of 0.5 to 0.6 X 104ml, a nurse culture system was essential to promote sustained divisions. With C. metuliferus, only first cell divisions were observed and there was no development of minicalli. When a mixture of these two species was plated out, division and regeneration of C. sativus was inhibited by the presence of C. metuliferus protoplasts (possibly due to a dilution of the plating density), but this was overcome by the presence of the C. Sativus nurse culture system (Table 1). When fusion treatments were imposed, their effects were determined on control protoplasts of C. sativus as well as in mixtures. Both PEG and high pH/Ca delayed the onset of divisions and development of minicalli in C. sativus, and this was partially overcome by the presence of the nurse culture (Table 1). Fusion frequencies were estimated to be around 5 to 6% for PEG and 2 to 5% for high pH/Ca. the presence of 1% DMSO in PEG was detrimental, since it caused cell enlargement and rupturing of the protoplast membrane. In mixtures of the two species with PEG or high pH/Ca as the fusigenic agent, (Fig. 1) cell divisions of fused and unfused cells were observed (Gif. 2) and minicalli developed (Fig. 3). These have been subcultured into callus proliferation medium containing full strength MS salts containing the same hormonal combinations, with 3% sucrose and 0.65% Phytoagar.
Discussion. Sustained division of mesophyll protoplasts to produce callus, which eventually gave rise to plantlets (unpublished) was accomplished for C. sativus but not in C. metuliferus. The lack of regeneration of C. metuliferus is advantageous in fusion studies, since only C. sativus-C sativus or C. sativus-C metuliferus fusions would be selected. Since low plating density affected the extent of protoplast divisions, which occurs because fusion followed by washings dilutes the initial plating density, a nurse culture system was employed in this study. Nutrients or compounds released by the adjacent growing cells enhanced division of mixed cells. Although protoplasts were used to provide the nursing effect, suspension culture cells can also be substituted (unpublished). The nurse culture system also minimized the extent of delay of cell divisions due to the fusion treatments.
PEG 8000 yielded higher fusion frequencies than high pH/Ca in this study. The callus developing from fusion mixtures would be of the C. sativus-C metuliferus hybrids. Because high plating densities are required for growth, individual isolation of potential hybrid cells cannot be accomplished. However, hybrid callus or plants regenerated from them should be distinguishable from C. sativus by morphological differences, chromosome numbers and isozyme banding patterns. The results described here are the first step toward accessing the desired traits of disease resistance from C. metuliferus.
Table 1. Response of protoplasts of Cucumis sativus and C. metuliferus, alone or in mixture, to a nurse culture and fusion treatments.
Species |
Fusion Treatment |
Days required to obtain Nurse culture |
Days required to obtain Cell wall formation |
Days required to obtain First division |
Days required to obtain Second-third division |
Days required to obtain Mini callus |
| C. sativus z |
No Fusion Treatment | – | 1-2 | 4-5 | 6-7 | 13 |
| C. sativus z |
No Fusion Treatment | + | 1-2 | 4-5 | 6-7 | 13 |
| C. sativusy | No Fusion Treatment | – | 3-4 | 10-20 | >20 | – |
| C. sativusy | No Fusion Treatment | + | 2-3 | 5-6 | 9-10 | 20 |
| C. metuliferusz | No Fusion Treatment | – | 5-6 | 8-10 | – | – |
| C. metuliferusz | No Fusion Treatment | + | 5-6 | 8-10 | – | – |
| Mixture | No Fusion Treatment | – | 3-4 | 10-20 | >20 | – |
| Mixture | No Fusion Treatment | + | 2-3 | 7-10 | 14 | 28 |
| C. sativus | PEG | – | 2-3 | 5-7 | 10-14 | 22-28 |
| C. sativus | PEG | + | 2-3 | 5-6 | 9-12 | 20 |
| C. sativus | High pH/Ca | – | 2-3 | 5-7 | 10-14 | 22-28 |
| C. sativus | High pH/Ca | + | 2-3 | 5-6 | 9-12 | 20 |
| Mixture | PEG | + | 3-4 | 7-8 | 12-16 | 28 |
| Mixture | High pH/Ca | + | 3-4 | 7-8 | 12-16 | 28 |
z Plating density of 2.5 to 3.0 X 104/ml.
y Plating density of 0.5 to 0.6 X 104/ml.
Fig. 1. Mixture of protoplasts of Cucumis sativus (S) and C. metuliferus (M).
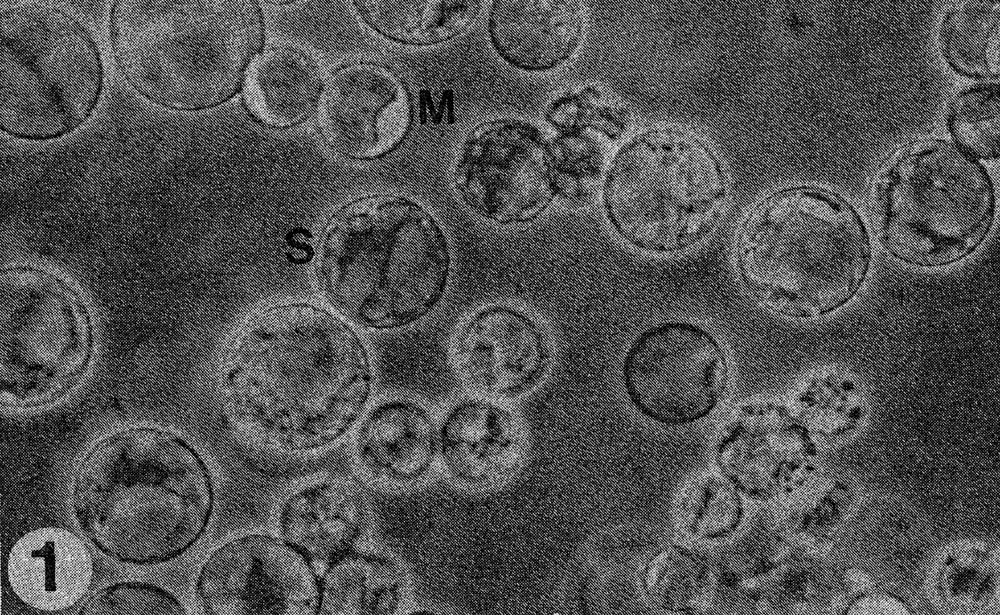
Fig. 2. Close-up of fusion of protoplasts of the two species.
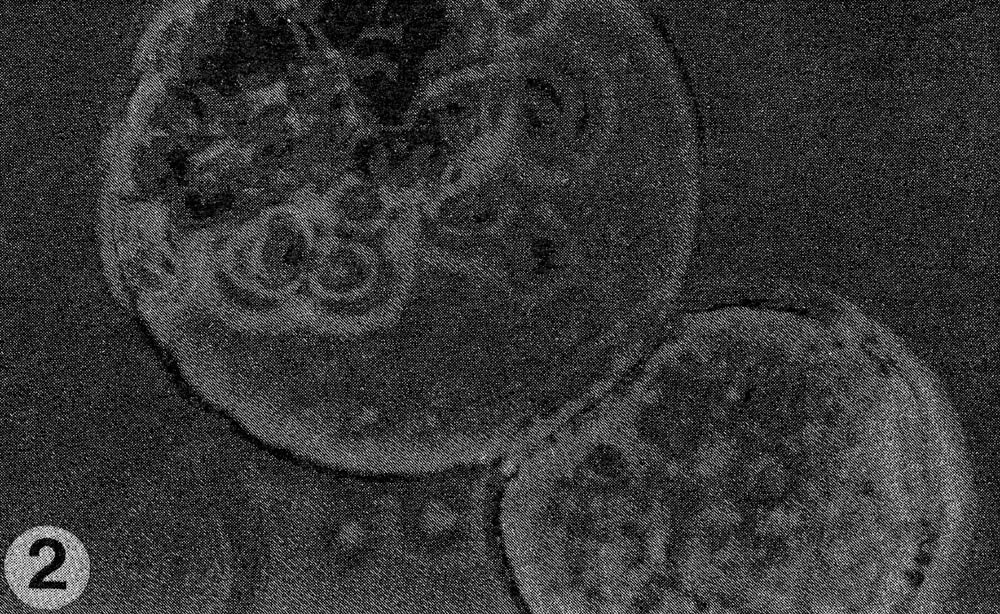
Fig. 3. Cell division of fused cells.
Literature Cited
- Austin, S., M.A. Baer and J.P. Helgeson. 1985. Transfer of resistance to potato leaf roll virus from solanum brevidens into Solanum tuberosum by somatic fusion. Plant Science 39:75-82.
- Austin, S., M.K. Ehlenfeldt, M.A. Baer and J.P. Helgeson. 1986. Somatic hybrids produced by protoplast fusion between S. tuberosum and S. brevidens: phenotypic variation under field conditions. Theor. Appl. Genet. 71:682-690.
- Deakin, J.R., G.W. Bohn and T.W. Whitaker. 1971. Interspecific hybridization in Cucumis. Econ. Bot. 25:195-211.
- Douglas, G.C., L.R. Wetter, C. Nakamura, W. A. Keller and G. Setterfield. 1981. Somatic hybridization between Nicotiana rustica and N. tabacum. III. Biochemical, morphological and cytological analysis of somatic hybrids. Can. J. Bot. 59:228-237.
- Dudits, D., G. Hadlaczky, E. Levi, O. Fejer, Z. Haydu and G. Lazar. 1977. Somatic hybridisation of Daucus carota and D. capillifolius by protoplast fusion. Theor. Appl. Genet. 51-127-132.
- Gibson, R.W., M.G.K. Jones and N. Fish. 1988. Resistance to potato leaf roll virus and potato virus Y in somatic hybrids between dihaploid Solanum tuberosum and S. brevidens. Theor Appl. Genet. 76-113-117.
- Granberry, D.M. and J.D. Norton. 1980. Response of progeny from interspecific cross of Cucumis melo x C. metuliferus to Meloidogyne incognita acrita. J. Amer. Soc. Hort. Sci. 105:180-183.
- Kao, K.N. and M.R. Michayluk. 1974. A method for high frequency intergeneric fusion of plant protoplasts. Planta 115:335-367.
- Keller, W.A. and G. Melchers. 1973. The effect of high pH and calcium on tobacco leaf protoplast fusion. Z. Naturforsch. 28c:737-741.
- Kho, Y.O., A.P.M. Den Nijs and J. Franken. 1980. Interspecific hybridization in Cucumis L. II. The crossability of species, an investigation of in vivo pollen tube growth and seed set. Euphytica 29:661-672.
- Kyozuka, J., Y. Hayashi and K. Shimamoto. 1987. High frequency plant regeneration from rice protoplasts by novel nurse culture methods. Mol. Gen. Genet. 206:408-413.
- Murashige, T. and F. Skoog. 1962. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 15:473-497.
- O’Connell, M.A. and M.R. Hanson. 1987. Regeneration of somatic hybrid plants formed between Lycopersicon esculentum and L. pennelli. Theor. Appl. Genet. 75:83-89.
- Primard, C., F. Vedel, C. Mathieu, G. Pelletier and A.M. Chevre. 1988. Interspecific somatic hybridization between Brassica napus and Brassica hirta (Sinapis alba L.). Theor. Appl. Genet. 75:546-552.
- Provvidenti, R. and R.W. Robinson. 1974. Resistance to squash mosaic virus and watermelon mosaic virus 1 in Cucumis metuliferus. Plant Dis. Reptr. 58:735-738.
- Punja, Z.K., F.A. Tang and L.H. Watkins. 1988. Identification of resistance to root know nematodes and virus diseases in Cucumis metuliferus and approaches to hybridization with Cucumis sativus by protoplast fusion. Phytopathology 78 (Abstr.)
- Shepard, J.F., D. Bidney, T. Barsby and R. Kemble. 1983. Genetic transfer in plants through interspecific protoplast fusion. Science 219:683-688.