Cucurbit Genetics Cooperative Report 17:40-49 (article 12) 1994
A. Baszczyk-Guzek and M. Szwacka
Department of Horticultural Plant Genetics and Breeding, Warsaw, and Agricultural University, Noworsynowska 166, 02-766 Warsaw, Poland
Cucumber is a species for which methods of regeneration of in vitro cultures have been elaborated (Malepszy, 1988; Malepszy et al., 1986). Tissue culture provides opportunities for selecting for resistance to different types of biotic and biotic agents. Thus far, mutants resistant to Fusarium oxysporum f. sp. cucumerinum (El-Kazzaz, 1990; Malepszy and El-Kazzaz, 1990) and herbicides, metribuzin or lunuron (Hasegawa, 1980), have been identified in suspension and callus culture. However, suspension culture variants resistant to streptomycin could not be regenerated (Malepszy, 1988). tolerance to salinity is a trait that can have practical implications for cucumber breeding. No individuals tolerant to salinity have been found among cucumis species (Anastasio et al., 1988). The following work attempts to obtain such forms using NaCl selection in tissue culture.
Methods. The highly inbred (S15) monoecious cucumber line of the variety ‘Borszczagowski’ (line B) was used for the experiments. The first and second leaves from 14 to 21 day-old seedlings were used as a source of explants, according to Malepszy (1988).
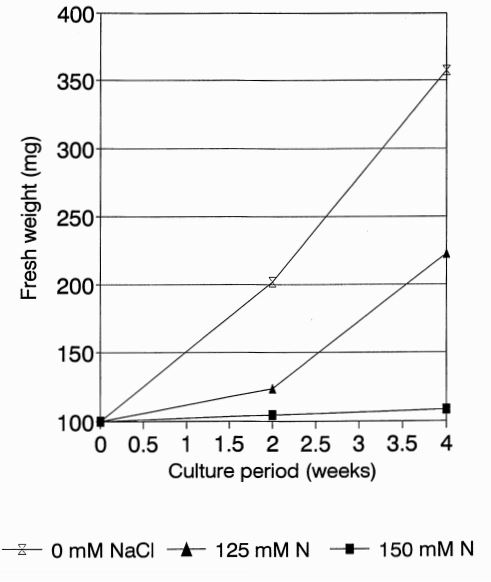
The culture medium used was based on Murashige and Skoog (1962) salts and vitamins enriched with 0.6 mg x dm-3 sucrose, 250 mg x dm -3 edamine and 8 g x dm -3 agar (medium) CSO). Callus pieces (~50 mg) were plated on culture media containing: 0. 10, 50, 100, 125, 150, 175 and 200 mM NaCl. A minimum of three replicates were made. Callus growth after 4 to 6 weeks of culture was established. Fresh weight of the calli on three different NaCl concentrations (), 125, and 150 mM was measured after 2 and 4 weeks. The data are presented in Figure 1 as the average of 4 replicates.
For selection of resistant variants, CSO medium was supplemented with two NaCl concentrations [125 mM (medium CS125) or 150 mM (medium CS150)] according to given selection parameters (Figure 2). Ten leaf discs (5 mm in diameter) were plated on one Petri dish supplemented with saline medium. In the first case, selection was accomplished by isolating some small pieces of callus (~2 mm2 ) after two weeks of culture from independent sites of an explant. Callus pieces were placed directly on the selection media or after one week preculture on control medium. In the second case, selection was initiated by isolating sectors of callus (i.e., yellow-green or mixed colors distinguishable from the remaining necrotic zones) after six to eight weeks of culture with or without a one week of preculture. The selected calli were taken through passages on salinized media, and the control line (line B) was transferred to NaCl – free medium. Each passage lasted 3 to 4 weeks.
Between the third and fourth passage after selection initiation, the selected calli were transferred on a different regeneration media without (R) or supplemented with two different NaCl concentrations (62.5 mM and 100 mM). The medium was the same as CSO, except that it lacked hormones. The control line was regenerated on medium R simultaneous with the production of selected calli lines. The calli were subcultured at two week intervals. Embryogenic regions and those regions upon which organogenesis occurred were chosen for transfer to fresh media. Once recovered, plants reached 5 to 7 cm in height at which time they were transferred to small pots containing a mixture of peat substrate and perlite, and placed in a growth room for 7 to 10 days to acclimate. After acclimation, plants were transferred to a greenhouse.
The influence of saline conditions on: 1) callus introduction of R1, R2 and F1 plants; 2) the relationship between photosynthetic parameters (efficiency of photosynthesis, stomatal conductance and efficiency of transportation); and, 3) R2 seedling growth was investigated. Six to twelve leaf explants from each of two selected R1 plants (53.8.3 and 61.6.3) or five from each of 18 to 20 of the control line (line B), F1 generations (line B x 53.8.3 and 53.8.3 x line B), and R2 generation (self pollination of 53.8.3 and 53.8.3 plant) were plated on NaCl-free medium (CSO) and saline conditions (CS125). After three passages (four weeks each) the growth of calli was estimated. R3 and F1 seeds were germinated at 25C in the dark on filter paper soaked with tap water. Three days later the germinated seeds were transferred into light (15 h photoperiod) and transplanted to a hydroponic system (half-strength of Murashige and Skoog salts). NaCl was absent or added at 75 mM to the nutrient solution.
A Li-6200 Portable Photosynthetic System was used to measure photosynthetic parameters (efficiency of photosynthesis, stomatal conductance and efficiency of transpiration). This system allowed for measurement of a leaf attached to a plant. the leaves (first, second and in one case third) of F1 seedlings treated with 75 mM NaCl were selected when they were almost fully expanded. Measurements were performed on the same leaves immediately after the transfer to saline or unsaline conditions (time t0 ) and after 3 (experiment I) or 5 (experiment II) days after NaCl treatment (time t1 ). Two leaves of each of two seedlings were included for photosynthetic measurements.
In another experiment, seeds were germinated for 36 hours in distilled water. Seven to ten seeds were used in each treatment. Seeds were sown in sand thoroughly soaked with salinized (125 mM NaCl) or unsalinized nutrient solution consisting of half-strength MS salts. The trials were covered with rain-out shelters to avoid unwanted precipitation. Every two days plants were supplemented with an equal amount of unsalinized nutrient solution. The measurements of plant length were performed after two weeks.
Results. In the initial callus growth experiment at NaCl concentrations up to 200 mM, the degree of salt stress was estimated based on visual symptoms. Although a strong inhibition of callus growth occurred at 125 mM, total suppression was observed at or above 165 mM. Starting from 125 mM callus greened rapidly (chlorophyll sectors distinguishable from the remaining necrotic zones). These green sectors never appeared in control callus. Figure 1 shows the growth of callus of line B at the different NaCl concentrations (0, 125 and 150 mM). After 2 weeks of culture at 125 mM or 150mM, less of an increase in fresh callus weight was observed when compared with control (0 mM). After 4 weeks of culture the callus exhibited a significant increase in fresh weight when grown in the absence of NaCl when compared to the remaining two concentrations. Differences in the increase of fresh calus weight between all three concentrations were significant (analysis of variance).
Figure 1. Fresh weight of cucumber callus line B after 2 and 4 weeks of culture.
For selecting salt tolerant cells two NaCl concentrations were chosen (125 mM and 150 mM). NaCl resistant variants with independent sectors of growing callus were primarily considered as a selection method (Figure 2). Initially, the number of initial resistant variants was less dependent on the method of selection. The NaCl concentration had a significant influence on growth but only in combinations without preculture (Table 1). In the eighth passage the number of variants decreased to zero in five out of eight combinations. After 11 weeks of culture only two variants in combination with preculture were maintained. Both the color and rate of growth were no homogenous. the occurrence of variously colored calli (from dark-green through yellow-green to yellow) and two-fold increases in fresh weight after two weeks of culture were noted. Until the eighth passage, the largest number of resistant variants were observed in the second method of selection, especially when the preculture was applied.
Figure 2. The ways of selection of cucumber callus resistant to NaCl.
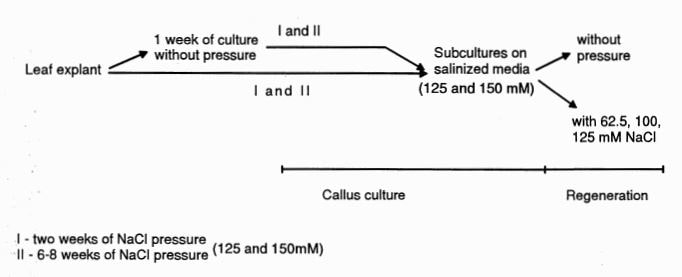
Table 1. The influence of selection method on the number of primary NaCl-tolerant cucumber cells variants.
No. of variants still tolerant after passages |
|||||||
Selection Methods¹ |
Medium² |
Initial no. of explants |
No. of primary tolerant variants |
Calli/explant |
4 |
8 |
11 |
I |
CS125 | 60 | 56 | 0.93 | 20 | 0 | 0 |
| P+CS125 | 72 | 78 | 1.08 | 28 | 0 | 0 | |
| CS150 | 40 | 15 | 0.38 | 11 | 0 | 0 | |
| P+CS150 | 50 | 36 | 0.72 | 11 | 2 | 2*4 | |
II |
CS125 | 73 | 46 | 0.63 | 14 | 2 | 0 |
| P+CS125 | 56 | 52 | 0.93 | 30 | 30 | 2**4 | |
| CS150 | 88 | 23 | 0.26 | 4 | 0 | 0 | |
| P+CS150 | 44 | 37 | 0.84 | 3 | 0 | 0 | |
1 I = 2 weeks of NaCl-pressure; II – 6-8 weeks of NaCl-pressure.
2 P = 7 days of preculture on NaCl-free medium.
3 * After this passage calli died.
4 Calli survived 15 passages.
Table 2. The reaction of elaf explants (determined as capable of callus growth) on CS0 and CS125 media after 4 weeks of culture.
No. of plants with callus regenerating explants on media |
|||
Line or cross |
No. of plants |
CSO |
CS125 |
| Line B | 8 | 8 | 0 |
| F1 [B x 53.8.3] | 12 | 11* | 6 |
| F1 [53.8.3 x B] | 8 | 8 | 3 |
| R2 [53.8.3. self] | 20 | 15* | 6 |
* Diminished through infections.
Table 3. The characteristics of photosynthesis in cucumber plants grown in nutrient solutin for 3 [I] or 5 [II] days.
A¹
|
B
|
C [MH2O)/m²/s] |
D [ppm] |
||||||
Line or cross |
NaCl Conc. |
t0 |
t1 |
t0 |
t1 |
t0 |
t1 |
t0 |
t1 |
| line B | 0 | 11.80 | 9.11 | 0.27 | 0.22 | 5.09 | 3.06 | 198 | 201 |
| [I] | 75 | 10.91 | 10.02 | 0.32 | 0.12 | 5.31 | 2.71 | 226 | 137 |
| R3 [53.8.3] | 0 | 5.68 | 7.14 | 0.28 | 0.18 | 5.23 | 3.65 | 255 | 215 |
| [I] | 75 | 4.66 | 7 | 0.27 | 0.13 | 4.43 | 3.76 | 256 | 197 |
| line B | 0 | 5.66 | 35 | 0.14 | 0.13 | 2.74 | 1.90 | 208 | 27 |
| [II] | 75 | 4.79 | 2.66 | 0.14 | 0.03 | 2.89 | 1.56 | 221 | 211 |
| F1 [53.8.3 x B] | 0 | 5.37 | 1.84 | 0.30 | 0.30 | 2.83 | 2.56 | 251 | 268 |
| [II] | 75 | 5.17 | 3.81 | 0.19 | 0.05 | 3.56 | 0.93 | 243 | 164 |
A¹ = efficiency of photosynthesis, B = stomatal conductance, C = efficiency of transpiration, and D = intercellular CO2 concentration.
Two types of conditions were tested for regeneration; NaCl-free medium and medium supplemented with NaCl. It was proven that regeneration in the presence of NaCl was impossible even at lower concentrations. Plants were regenerated from compact structures (yellow or yellow-green callus). Symptoms of callus destruction became visible after three weeks of culture on R medium.Of 51 calli plated on R medium, only two showed further development and initiation of regenerating sectors. These sectors were subcultured on fresh R medium. After five weeks, the regeneration of shoot-like structures was observed. Later, plantlets with leaflets were also observed. Finally, after eight weeks of culture on medium R, 15 plants were regenerated. Only four of these survived transfer to soil and two adapted to greenhouse/field culture conditions. They were designated 53.8.3 and 61.6.3. Plant 61.6.3 differed phenotypically from the control plant; its growth was not more than 13 cm in height. Although one female flower appeared on this plant, fruit were not produced. Viability of pollen was apparently lower (53%) relative to the control and plant 53.8.3. No regenerants from initially resistant callus were derived from selection method 1.
The stability of the selected trait was tested by comparing the calli induced from control and those which resulted from tolerant plants. Leaf explants of R1 plants were capable of developing callus on media (CSO and CS125). On the CS125 medium, callus survived at least three passages and calli assumed a yellow coloring (plant 53.8.3). However, the callus of plant 61.6.3 apart from its color (dark-green) also differed from the others in its reaction under unsalinized conditions. It survived on NaCl-free medium for only two passages. In contrast to some explants of F1 and R2 plants, leaf explants of control plants (line B) did not regenerate callus on CS125 medium during four weeks of culture (Table 2). In the self pollinated progeny of regenerant 53.8.3 (R2) somewhat resistant plants were recorded (30%). However, in the F1 generation approximately 50% of the resistant plants were independent of the cross direction.
The aim of the experiments presented in Table 3 was to examine the effect of salt stress on photosynthesis. The data presented show that only the leaves of control plants treated for 5 days had decreased CO2 -assimilation rates. The intercellular CO2 concentration measured was in the rate of 137 to 271 ppm, and the lowest concentration was sufficient for efficient photosynthesis. An addition of 75mM NaCl to the nutrient solution resulted in stomatal closure in leaves of control and F1 plants. The transpiration of F1 plants was affected by salt amendments only in one case.
The growth characteristics and segregation data for F2 plants are shown in Table 4. Growth in 125 mM NaCl decreased the height of salt tolerant plants by about 30% relative to the control (0 mM NaCl). The growth of sensitive plants under salt stress was totally inhibited. The pattern of segregation in both F2 crosses showed that 40% of the F2 seedlings were tolerant to the salt concentrations administered in this study.
Discussion. Developing crops with enhanced salt tolerance is an important current goal in our laboratory. No real source of salt tolerance has been found within Cucumis (Anastasio et al. 1988; Shannon and Francois, 1978). Experimental data presented by Shannon and Francois (1978) support the idea that only a partial degree of tolerance is available in the genetic pool of C. melo. One possibility for recovering lines with increased salt tolerance through selection in vitro. It is known that salt tolerance is not stable (Hasegawa et al., 1980). Therefore, an analysis of the resistance mechanism on cellular level has been evaluated using resistant cells obtained from stepwise selection after a long culture period (Hasegawa, 1980; Watad et al., 1983, 1985). No plants were regenerated and analyzed in these experiments. In the present study two methods of selection were applied – one with short term (2 weeks) and the other with long term (6 – 8 weeks) salt exposure. Both approaches used two NaCl concentrations. The lower salinity levels decreased fresh callus weight (~80%), and higher levels were lethal. Similar selection methods were done by Narayanan and Rangasamy (1989). In the present study plants were regenerated only from calli selected after long term exposure to salt. However, the number of resistant calli at the last selection step was very low regardless of selection method. Therefore, It is concluded that selection efficiency was dependent on the selection method.
The conditions for cucumber in vitro regeneration are well known (Malepszy, 1988). However, under salinity stress some differences were found. First, the selected callus was green. This condition was never observed under normal conditions. Second, regeneration was not possible on the saline medium since the concentration was two times lower than that used in the first selection method. Although a negative influence of salinity stress on regeneration has been observed by Nabors et al. (1980), the opposite was observed by Galiba and Yamada (1988). In our studies, regenerated cucumber plants had difficulties adapting to normal growth conditions and the regenerant of one line was unable to seed set (self sterility and sterile pollen). This was similar to cucumber plants regenerated after selection on herbicides (Malepszy et a., 1991) and Fusarium oxysporum (El-Kazzaz, 1990; Malepszy and El-Kazzaz, 1990). In the later, regenerant plants were male fertile.
The reaction of regenerated plants to salinity was analyzed to salinity was analyzed by comparing photosynthetic parameters and growth responses. The degree of salinity applied in the first series of experiments was chosen to be 75mM, according to the results of Drew et al. (1990) who showed inhibition of cucumber photosynthesis at 50 mM. Our results indicate that photosynthetic and transpiration rates could not serve as reliable criteria for salinity stress discrimination. This may be due to differential cultivar sensitivity in their experiments as well as ours. Salinity is known to reduce the growth of salt sensitive species. We observed that salt had little effect on the growth of F2 plants.
Plants produced in our studies tolerated salinity differentially: 1) callus of R1 plants was resistant: 2) photosynthetic C02 fixation and stomatal conductance of R3 plants were not affected by salinity; and 3) some F1 and F2 plants were sensitive and tolerant. The mode of inheritance was not determined, but F1 phenotypic observation suggests dominant gene action for salt tolerance, similar to tobacco (Nabors et al., 1980). In contrast, tolerance in rice is recessive and conditioned 2 genes (Narayanan and Rangasamy, 1989). In another study (Kononowicz et al., 1990) all tobacco plants regenerated from salt tolerant selection experiments were hexaploid, suggesting that hexaploid cells were more likely to regenerate than cells of other ploidy levels. The results of our work indicate that NaCl-tolerant cucumber can be selected using in vitro culture and that the developed procedure can be applied in a plant improvement program.
Table 4. Seedling tolerance to NaCl within F2 cucumber progeny.
Line or cross |
NaCl concent [mM] |
No. seedlings tested |
Av. length of seedlings [cm] |
Tolerant to sensitive seedlings ratio |
Percent of tolerant seedlings |
| line B | 0 | 10 | 3.62 | — | — |
| 125 | 10 | 0.00 | 0 | 0 | |
| F2[Bx53.8.3] | 0 | 7 | 5.22 | — | — |
| 125 | 10 | 3.40* | 4/6 | 40 | |
| F2[5.38.3xB] | 0 | 7 | 3.81 | — | — |
| 125 | 10 | 2.25* | 4/6 | 40 |
*Average length of tolerant variants.
Literature Cited
- Anastasio, G., M.S. Catala, G. Palomares, J. Costa and F. Nuez. 1988. Salt tolerance in wild cucurbits. Proc. EUCARPIA Meeting on Cucurbit Genetics and Breeding. Avignon-Montfavet, May 31-June 1/2, pp. 175-180.
- Drew, M.S., P.S. Hole and G. A. Picchioni. 1990. Inhibition by NaCl of C02 fixation and yield of cucumber. J. Amer, Soc. Hort. Sci. 115:472-477.
- El-Kazzaz, A. 1990. In vitro selection of cucumbers resistant to Fusatium oxysporum. Doctoral Thesis, Warsaw Agricultural University, Faculty of Horticulture.
- Galiba, G. and Y. Yamada. 1988. A novel method for increasing the frequency of somatic embryogenesis in wheat tissue culture by NaCl and KCl supplementation. Plant Cell Rpts. 7:55-58.
- Hasegawa, P.M., R.A. Bressan and A.K. Handa. 1980. Growth characteristics of NaCl-selected and nonselected cells of Nicotiana tabacum. Plant Cell Physiol. 21:1347-1355.
- Kononowiez, A.K., P,M. Hasegawa and R.A. Bresan. 1990. Chromosome number and nuclear DNA content of plants regenerated from salt adapted plant cells. Plant Cell Rpts. 8:767-679.
- Malepszy, S. 1988. cucumber (Cucumis sativus L.), In: Y.P.S. Bajaj (ed.) Biotechnology in Agriculture and Forestry 6:277-293, Springer Verlag, Berlin-Heidelberg.
- Malepszy, S. and El-Kazzaz. 1990. In vitro culture of Cucumis sativus. XI. Selection of resistance to Fusarium oxysporum. Acta Hort. 280:455-458.
- Malepszy, S., A. Nadolska-Orczyk and W. Orcszyk. 1986. Systems for generation of Cucumis sativus plants in vitro. In: Genetic Manipulation in Plant Breeding. Hom, Jensen, Odenbach, Schieder (eds.) pp. 429-432.
- Malepszy, S., K. Witkowski and S. Zgagacz. 1991. Cucumber (Cucumis sativus L.) variants resistant to metribuzin or linuron are not viable. Cucurbit Genet Coop. Rpt. 14:34-42.
- Murashige, T. and F. Skoog. 1962. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiologia Plant. 15:473-497.
- Nabors, M.W., S.F. Gibbs, C.S. Bernsteinand M.E. Meis. 1980. NaCl-tolerant tobacco plants from cultured cells. Z. Pflanzenphysiol. 97:13-17.
- Narayanan, K.K, and S.R.S. Rangasamy. 1989. Inheritance of salt tolerance in progenies of tissue culture selected variants of rice. Current Science 58:1204-1205.
- Shannon, M.C. and L.E. Francios. 1978. Salt tolerance of three musk melon cultivars. J. Amer. Soc. Hort. Sci. 103:127-130.
- Watad, A.A., H.R. Lerner and L. Reinhold. 1985. Stablity of the salt-resistant character in Nicotiana cell lines adapted to growth in high NaCl concentrations. Physiol. Veg. 23:887-894.
- Watad, A.A., L. Reinhold and M. Lerner. 1983. Comparison between a stable NaCl-selected Nicotiana cell line and the wild type. Plant Physiol. 73:629-642.
Acknowledgements. The authors wish to thank Professor Zofia Stark and Doctor Barbara Wawrzonowska for help in the measuring of phyotosynthetic parameters.
This work was partly financially supported by Polish government grant C.B.P. 04.12.3.9.