Cucurbit Genetics Cooperative Report 20:21-23 (article 11) 1997
G. Caglar
Department of Horticulture, Faculty of Agriculture, University of K.S.U., 46060, Kahramanmaras, Turkey
K. Abak
Department of Horticulture, Faculty of Agriculture, University of Cukurova, 01330, Adana, Turkey
Introduction: Recently, haploid cucumber plants (n=7) have been obtained from in vitro haploid embryo culture. The recovery of haploid cucumber plants has been sufficient for their use in breeding programs (1). Although haploid plants can be used directly in genetic analysis, they should be converted to dihaploid plants (2n=14) via chromosome doubling in order to develop cultivars.
Colchicine has been effective for chromosome doubling in many species. Colchicine has been applied in situ or in vitro at various doses and durations depending upon the species used. Although in situ colchicine application often results in cytochimera formation (2), in vitro colchicine application can reduce chimera formation frequency (3). The objective of this research was to determine a suitable dose and duration for in vitro colchicine application in cucumber to obtain dihaploid plants.
Material and Methods: One month old in vitro cucumber plantlets (roots and some leaves removed) were treated by immersion in aqueous colchicine solution at 0.5% or 1% for 2 or 4 hr. The plantlets were then rinsed three times in sterile distilled water and laid on sterile paper towel to remove excess water. Since they were directly in contact with the colchicine solution, the basal and apical ends of the plantlets were trimmed slightly. These micro-cuttings possessed a single node and were transferred to test tubes containing E20A media. The healthy plants were acclimatized, and transferred to a mixture of peat and volcanic tuft under misting. The surviving plants were then planted in soil in a greenhouse. The ploidy levels of the established plants were determined by chromosome counting using root tips to verify the effectiveness of colchicine treatments.
Results and Discussion: The results of the experiment are summarized in Table 1. The percentage of surviving and healthy developing plantlets decreased after colchicination at the higher dose or longer duration. Water drops on microcuttings after rinsing in distilled water resulted in contamination that led high plantlet losses. Therefore, drying after rinsing is crucial for obtaining a high percentage of healthy plantlets.
The shoot growth of some colchicine treated plantlets was limited, and the period from colchicine application to recovery of the first micro-cuttings was longer at the higher dose (1%) than at the lower dose (0.5%).
The number of micro-cuttings per plant after 4 months (by two successive micro-cutting regenerations) ranged from 2.7 to 5.0. A total of 336 plants were obtained by micro- cuttings in this experiment. However, most of the transplants did not survive under greenhouse mist culture. Only 37 plants were established in soil in the greenhouse. The established haploid and dihaploid plants were grown for 5 months, and their ploidy levels were confirmed by their morphological features. Seeds were also extracted from fruits of dihaploid plants.
Treatment of plantlets with 0.5% colchicine for 2 hr had no effect on the production of dihaploids. We obtained dihaploids at a rate of 53% following treatment of plantlets with 0.5% dose for 4 hr, and 60% with treatment of 1% colchicine for 2 hr. Although colchicine applications with 1% dose for 4 hr produced only dihaploids, the number of the resulting plants was low (3 plants). This might be related to the higher percentage of unhealthy plants. In the light of these findings, it appears that dihaploid cucumber plants can be derived from haploids either by the application of colchicine with 0.5% dose for 4 hr or 1% dose for 2 hr.
Table 1. The development of cucumber plantlets and their ploidy levels after in vitro colchicination.
Colchicine doses (%) and durations (hr) |
||||
0.5% |
1.0% |
|||
Plant development |
2 hr |
4 hr |
2 hr |
4 hr |
| Number of microcuttings planted | 41 | 49 | 38 | 38 |
| Percentage of surviving plantlets | 92.7 | 59.2 | 55.3 | 42.2 |
| Percentage of healthy developing plantlets | 68.2 | 51.0 | 55.3 | 36.8 |
| Days for the first microcuttings | 51 | 51 | 54 | 54 |
| Number of microcutting per plant (in 4 months after colchicination) | 5.0 | 3.8 | 2.7 | 4.1 |
| Number of transplants | 100 | 88 | 54 | 94 |
| Number of haploid plants | 5 | 9 | 4 | – |
| Number of dihaploid plants | – | 10 | 6 | 3 |
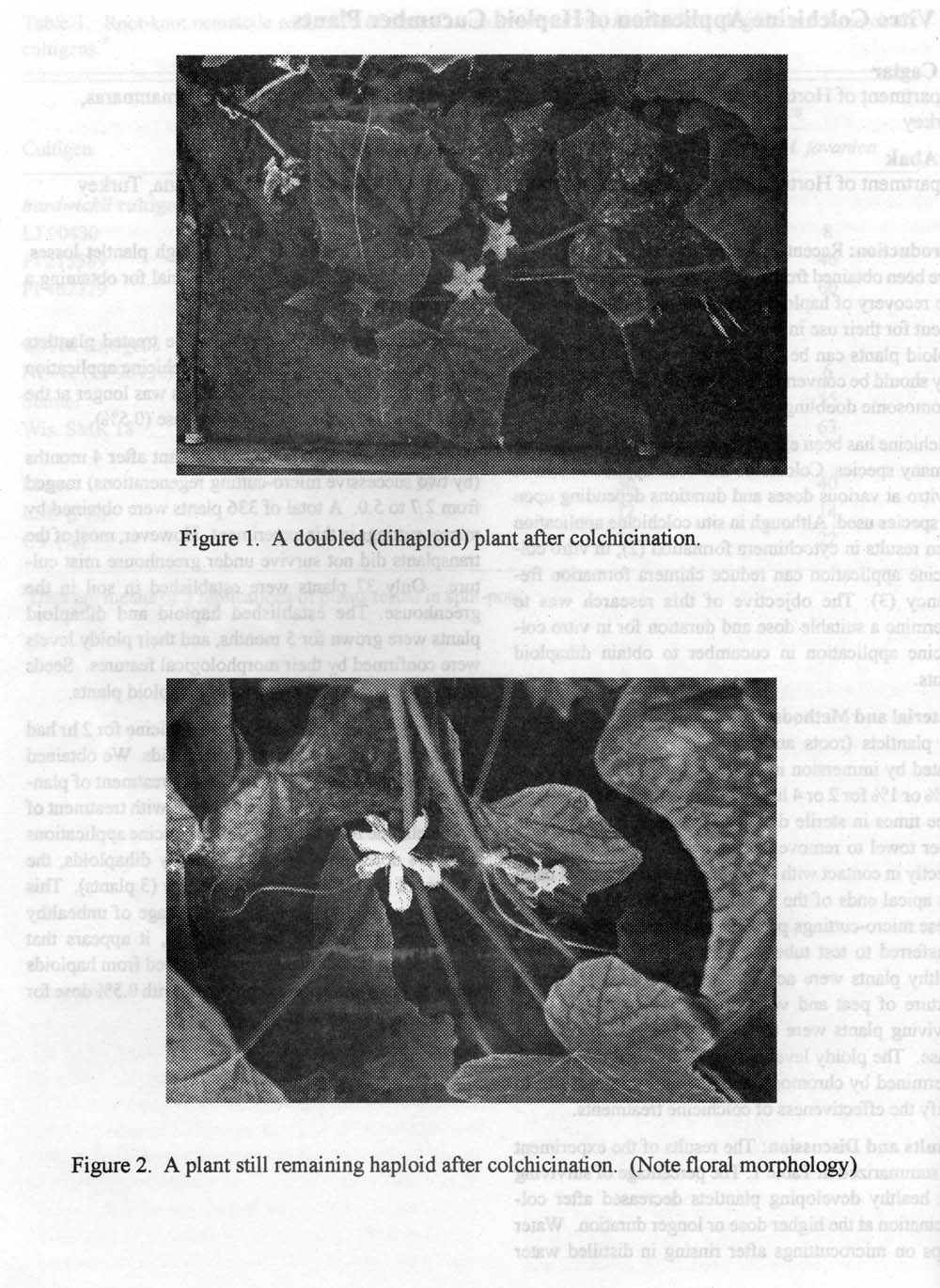
Figure 1. A doubled (dihaploid) plant after colchicination.
Figure 2. A plant still remaining haploid after colchicination. (Note floral morphology)
Literature Cited
- Caglar, G. and K. Abak. 1996. Efficiency of haploid production in cucumber. Cucurbit Genetics Coop. Rept. 19:36-37.
- Hermsen, J.G.T. and M.S. Ramanna. 1981. Haploidy and plant breeding. Phil. Trans. R. Soc. Lond. B. Vol. 292, 499-507.
- Pierik, R.L.M.. 1989. In Vitro Culture of Higher Plants. Martinus Nijhoff Publ., Dordrecht, 344 p.