Cucurbit Genetics Cooperative Report 22:24-27 (article 10) 1999
Patrick J. Conner and Timothy J. Ng
Dept. Natural Resource Sciences, University of Maryland, College Park, MD 20742-4452 USA
Introduction
Mature melons become soft and dehydrated, even when storied in cool humid conditions. the short time inwhich fruits must be harvested, sold and consumed is a serious constraint to profitability. Studies on the timing and nature of ripening-associated events, which may lead to the production of new cultivars with improved storage ability or the development of better post-harvest handling techniques, are of critical importance to the industry.
Lipid peroxidation, a prominent feature of plant senescence and aging (Kumar and Knowles, 1993),may impair membrane structure and function (Thompson et al, 1997). Lipid peroxidation is a consequence of metabolic processes in plant cells which produce reactive oxygen species (ROS) such as superox9ide, hydrogen peroxide, and singlet oxygen. For every source of lipid peroxidation in the plant cell, there are corresponding defense mechanisms. Among the most important non-enzymatic defense mechanisms are the water-soluble reductants glutathione (GSH) and ascorbate (ASC), and the lipid-soluble vitamin e (Tocopherol). Vitamin E protects against oxygen radicals that initiate lipid peroxidation and serves as a scavenger of chain-propagating free radicals such as lipid peroxyl radicals (Winston, 1990). ASC and GSH are proposed to be direct free-radical scavengers in the cytoplasm and may act synergistically with vitamin E in the inhibition of oxidative damage to cell membranes.
Melon fruit show a progressive increase in membrane permeability, as measured by electrolyte leakage, as the fruit matures (Lester, 1988). this leakage proceeds most rapidly in the interior of the fruit, which ripens sooner. In a comparison between short-and long-storage life nonnetted melons electrolyte leakage increased with ripening and was always higher in the short-storage cultivar, whereas the long-storage life cultivar had little increase in membrane permeability as the fruit ripened (Lascan and Baccou, 1996). The loss of membrane integrity was associated with a breakdown of phospholipids. Membrane integrity thus seems to be an integral component of melon fruit ripening. The current study was aimed at evaluating lipid peroxidation and status of the antioxidants vitamin E, GSH and ASC during melon fruit development and senescence.
Methods
Plant material ‘Perlita’ melon were grown singly in pots in a greenhouse using a trellis system. Hermaphroditic flowers were pollinated and tagged at anthesis, with one to two fruits per plant were allowed to develop. Unripe fruit were harvested at 20, 30, and 40 days post0anthesis (PA) . Ripe fruit were harvested at the full-slip (FS) stage, which occurred from 42 to 49 days PA, and were either sampled that day or stored at 20 C and ambient humidity for 5 or 10 days. Thus, samples were taken at six different developmental stages, and four to six fruits were sampled at each stage.
For analyses, the epidermis was removed from the fruit and tissue was extracted from the endo-mesocarp (E/M), middle-mesocarp (M/M), and hypodermal-mesocarp (H/H) regions of the fruit. H/M was tissue up to 5 mm below the epidermis, E/M was taken from the mesocarp within 5 mm of the seed cavity, and M/M tissue was central to the exterior and interior of the fruit. Tissue sample were collected and frozen in liquid N2, ground to a fine powder with dry ice, and stored at -80 C.
Chemical analyses. As a measure of lipid peroxidation, malondiadehyde (MDA) content was assayed by HPLC using the procedure of Iturbe-Ormaetxe et al. (1988). Vitamin E was assayed via a reverse-phase HPLC using the procedure of Spychalla and Desborough (1990). Reduced ascorbate determination was based on the reduction of Fe3+ to Fe2+ by ASC in acidic solution. The Fe2+ then forms a complex with bipyridyl, giving a pink color that was measured with a spectrophotometer (Law et al., 1983. Reduced glutathione was measured by a HPLC after derivation with 5,5′-ditho-bis-(2-nitrobenzoix acid) (Ranieri et al., 1993)
Results
As melons reach their climacteric peak, an abscission layer forms between the fruit and the vine a stage often referred to as full-slip (FS). The GS stage was reached at an average of 45 days PA in this study. At 20 days PA, melon flesh was quite firm and uniformly green. At 30 days PA, the E/M tissue had begun to show signs of the characteristic orange color. M/M tissue was orange at 40 days PA, but firmness was not significantly different from 20 and 30 days PA. At FS, fruit firmness was approximately half of the unripe values (Data not shown). Fruit firmness continued to decline at FS+5 and FS+10 days, at which point fruits were soft and watery and the hypodermis had begun to exhibit necrotic spots.
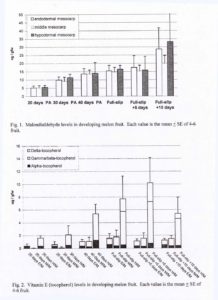
MDA, a breakdown product of lipid hydroperoxides, was used as an indicator of lipid peroxidation. MDA content was similar for all three tissues types at a given stage of development (Fig. 1). MDA content increased throughout development, most dramatically in the period from FS+5 days to FS+1- days.
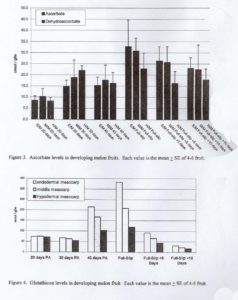
Three toicopherol (vitamin E) isomers ( α , β / γ and δ ) were detected in the chromatograms of melon tissues. Reverse-phase HPLC cannot separate the β and γ isoforms, so this peak was designated as β / γ. Total tocopherol concentration increased during development to maximal levels at FS or FS+5 days depending on tissue type (Fig. 2). Tocopherol levels were always several-fold higher in the H/M tissue as compared to E/M and M/M tissue. ASC concentrations increased slightly fro 20 to 40 days PA, the increased up to 3-fold in the period from 40 days PA to FS, and declined thereafter (Fig. 3). GSH concentrations were similar at 20 and 30 days PA, increased in the period from 30 days PA to FS, and then decreased to their lowest level at FS+10 days (Fig. 4).
Discussion
ROS levels tend to increase when plants are exposed to stress (Shewfelt and Purvis, 1995). The peroxidation of lipids is the most frequently cited effect of this increase within the plant cell (Winston, 1990). The increasing levels of MDA in ripening melon fruits provides additional evidence of peroxidation and breakdown of lipids. This is consistent with previous studies showing increasing electrolyte leakage as melon fruits mature (Lester,1988).
The balance from an anti-oxidant state to a pro-oxidant state in the cell can be triggered by an increase in ROS formation, a decrease in a defense mechanism, or a combination of the two. The concentration of all three antioxidant levels coincided with a period from 40 days PA to FS+5 days, when MDA levels were relatively stable, suggesting that the tissue was successfully coping with the oxidative stress. However, in the senescing tissue from FS+5 days to FS+10 days, MDA levels again increased, indicating new lipid peroxidation. While ASC and vitamin E levels were roughly steady during this period, GSH declined dramatically. This may indicate a shift to a more pro-oxidant state leading to lipid peroxidation.
Literature Cited
- Kumar, G. and N. Knowles. 1993. changes in lipid peroxidation and lipolytic free-radical scavenging enzyme activities during aging and sprouting of potato (Solanum tuberosum) seed-tubers. Plant Physil. 102:115-124.
- Lacan, D. and J. Baccou. 1996. Changes in lipids and electrolyte leakage during nonnetted muskmelon ripening. J. Amer. Hort.Sci. 121:554-558.
- Lester, G. 1988. Comparisons of ‘Honey Dew’ and netted muskmelon fruit tissues in relation to storage life. HortScience 23:180-182.
- Law, M., S. Charles and B. Halliwell. 1983. Clutathione and ascorbic acid in spinach (Spinacia oleracea) chloroplasts. Biochem. J. 210:899-903.
- Iturbe-Ormaetxxxe, I. P. Escuredo, C. Aarrese-Igor and M. Becana,. 1998. Oxidative damage in pea plants exposed to water deficit or paraquat. Plant Physiol. 116:173-181.
- Ranieri, A., G. D’Urso, C. Nali, G. Lorenzini and G. Soldatini. 1996. Ozone stimulates apoplastic antioxidant systems in pumpkin leaves. Physiol. Plant. 97:381-387.
- Shewfelt, R. and A. Purvis. 1995. Toward a comprehensive model for lipid peroxidation in plant tissue disorders. HortScience 30:213-218.
- Spychalla, J. and S. Desborough. 1990. superoxide dismutase, catalase, and -tocopherol content of stored potato tubers.
- Thompson, J., C. Froese, Y. Hong, K. Hudak and M. Smith. 1997. Membrane deterioration during senescence. Can. J. Bot. 75:867-869.
- Winston, G. 1990. Physiochemical basis for free radical formation in cells: Production and defenses. In: Alscher, R.G. and J.R. Cummings (eds.). Stress Responses in Plants: Adaptation and Acclimation Mechanisms. Wiley-Liss, New York.