Cucurbit Genetics Cooperative Report 23:8-11 (article 3) 2000
G. Fazio and J.E. Staub
USDA-ARS Vegetable Crops Research Unit, University of Wisconsin-Madison, Department of Horticulture, 1575 Linden Dr., Madison, WI 53706
Introduction. The acreage planted for mechanically-harvested cucumbers in the United States has increased 30 to 40% in the last 20 years. Because of rising labor costs and contract disputes this trend is projected to continue (Merchandising Guide, 1988). In the northern United States, acreage dedicated to once-over mechanical harvest is significant. For example, in Michigan, Wisconsin, Delaware, New York, Washington and Oregon mechanically harvested acreage ranges between 30 to 45%.
Curiously, the yield of pickling cucumber (gynoecious or G, and monoecious or M) has plateaued in the last 15 years. Studies by Widders and Price (1989) and Staub et al. (1989 and 1992) suggest that this recent plateau may be associated with net photosynthetic capacity. Resource limitations may explain why fruit developing from the first pollinated flower on each lateral branch inhibits the development of subsequent fruits (Denna, 1973; Fuller and Leopold, 1977).
To overcome the yield plateau and to respond to the need for cultivars suitable for mechanical harvesting, our breeding program is manipulating cucumber plant architecture to develop high yielding genotypes. Standard cucumber varieties (G x M or G x G hybrids) posses an indeterminate (De) plant habit and few lateral branches (`1 to 2). We are developing all female genotypes which are short in stature (determinate; de) and possess a multiple lateral branching habit (`5 to7 branches). This plant type can be sown at relatively high densities (compared to standard indeterminate types), and allows for an increase in early, concentrated yield (Staub et. al., 1992).
Information on quantitatively inherited traits related to yield components, however, is spare. Serquen et al. (1997) suggest that few genes (perhaps 5 to 8) control days to anthesis, sex expression, mainstem length, numbers of multiple lateral branches, and fruit number and weight. We are particularly interested in multiple lateral branching since this trait is highly correlated to yield response (). We present herein response to phenotypic selection in populations segregating for multiple lateral branches in the cross between line G421 (G, de, x H-19 (M, De). This experiment is part of a larger experiment that aims to compare the efficiency of marker-assisted selection of MLB to phenotypic selection in this cross.
Materials and Methods:The gynoecious determinate cucumber inbred line G421 possessing normal-sized leaves and low lateral branch number (approximately 1) was crossed with the monoecious indeterminate little leaf inbred line H-10 possessing high lateral branching (approximately 8) in the winter of 1997 to produce F1 progeny. In the spring of 1998, 15 F1 plants were used as males (after sex expression change) to pollinate 200 G421 plants to generate BC1 seed (greenhouse in Arlington, Wisconsin).
To change sex expression, apical meristems of selected plants were treated3 times in 5 day intervals with 2-3 ml aerosol solution of 6 mM silver thiosulfate. Selected plants were then selfed and backcrossed to field-grown G421 plants (recurrent parent).
In the summer of 1999, seed of selected BC2 (3233) and BC1S1 (1,367) from summer 1998 as well as parents, F1 hybrids (36), F2 (400), and BC1 (397) progeny seed lots were planted at Hancock, Wisconsin (Table 1). Parents, F1 , F2, BC1, BC1S1and BC2 families were arranged in a randomized complete block design with three replications of 12 plants each (total of 36 plants per family). Seed was planted 0.45 cm apart on rows positioned 1.5 m apart. Data collection, sex conversion, and selection were performed similar to summer 1998. Selections we backcrossed to G421.
Expected gain from selection was calculated according to Falconer and Mackay (1996) as R=1/2ih2 ơ P .The intensity of selection, i was adjusted to 1.2 of its tabulated value to account for the fact that no selection was applied to the females (G421). The mean h2 (0.45) used for calculation was taken from a previously published study that estimated heritabilities for this trait in two locations (Wisconsin and Georgia) (Serquen et al., 1997).
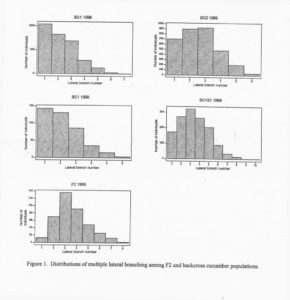
Results and discussion:The mean MLB number of the 126 selected plants in the BC1 population was selected, the expected gain from selection was calculated to be 0.57 units. The realized gain from selection based on the difference of the generation means was approximately 0.4 units. the replications of the base BC1 population across years were similar. Means, standard deviations and population numbers are given in Table 1. The distributions of multiple lateral branches differ in BC1 (MLB mean – 2.2), BC2 (MLB mean = 2.6), and BC2S1(MLB ,mean 3.4) populations (Figure 1). The progressive changes (increase in MLB) in these generations are the results of selection and increased homozygosity ( BC1S1 ; fixation) of loci affecting this trait.
A subset of approximately 200 BC2 plants (MLB mean = 516) of the BC2 population were selected to produce BC3 families. As a result of this selection experiment we now possess approximately 150 BC3 families representing independent recombination events. These families will be used to generate either nearly isogenic lines (Bernacchi et al., 1998) or congenic lines (Hill, 1998) for detailed QTL mapping and to increase our understanding the genetic control of MLB in cucumber.
Table 1. Generation means for multiple lateral branching in cucumber.
| Generation | Year | Mean | SD | N |
| BC1 | 1998 | 2.23 | 1.20 | 2985 |
| G421 | 1999 | 1.75 | 0.84 | 150 |
| H19 | 1999 | 7.90 | 1.60 | 36 |
| F1 | 1999 | 4.61 | 0.98 | 36 |
| BC1 | 1999 | 2.21 | 1.14 | 397 |
| BC2 | 1999 | 2.60 | 1.21 | 3233 |
| BC2S1 | 1999 | 3.38 | 1.616 | 1367 |
| F2 | 1999 | 3.58 | 1.38 | 400 |
Literature Cited
- Bernacchi, D., T. Beck-Bunn, D. Emmartty, Y. Eshed, S. Inai, J. Lopez, V. Petiard, H. Sayama, J. Uhlig, D. Zamir, and S.D. Tanksley. 1988. Advanced backcross QTL analysis of tomato. II. Evaluation of near-isogenic lines carrying single-donor introgressions for desirable wild QTL-alleles derived from Lycopersicon hirsutum and L. piminellifolium. Theor. Appl. Genet. 97:170-180.
- Denna, D.W. 1973. Effects of genetic parthenocarpy and gynoecious flowering habit on fruit production and growth in cucumber, Cucumis sativus L. J. Amer. Soc. Hort. Sci. 98:602-604.
- Falconer, D.S. and F.C. Mackay. 1996. Introduction to quantitative genetics. Harlow Essex, England, Longman LTD.
- Fuller, G.L. and C.A. Leopold. 1977. The role of nucleic acid synthesis in cucumber fruit set. J. amer. Soc. Hort. Sci. 102:384-388.
- Hill, W.G. 1998. Selection with recurrent backcrossing to develop congenic lines for quantitative trait loci analysis. Genetics 149:1341-1352.
- Kuper, R.S. and J.E. Staub. 1988. Combining ability between lines of cucumis sativus L. and Cucumis sativus var. hardwickii (R.) Alef. Euphytica 38:197-210.
- Merchandising Guide. 1988. Pickle Packers International, Inc. St. Charles, IL.
- Serquen, F.C., J. Bacher, and J.E. Staub. 1997. Genetic analysis of yield components in cucumber at low plant density. J. Amer. Soc. Hort. Sci. 122:522-528.
- Staub, J.E. 1989. source-sink relationships in cucumber. Cucurbit Gen. Coop. Rpt. 12:11-14.
- Staub, J.E., L.D. Knerr and H.J. Hopen. 1992. Effects of plant density and herbicides on cucumber productivity. J. Amer. Soc. Hort. Sci. 117:48-53.
- Widders, I.E. and H.C. Price. 1989. Effects of plant density on growth and biomass partitioning in pickling cucumbers. J. Amer. Soc. HOrt. Sci. 11:751-755.