Cucurbit Genetics Cooperative Report 13:8-9 (article 3) 1990
Todd C. Wehner and S. Alan Walters
Department of Horticultural Science, North Carolina State University, Raldigh NC 27695-7609
Kenneth R. Barker
Department of Plant Pathology, North Carolina State University, Raleigh NC 27695-7616
In determining resistance of plant cultigens to root knot nematodes (Meloidogyne spp.), eggs are often used as inoculum. It is important to know what level of inoculum to use on a given cultigen (accession, cultivar, breeding line, etc.) In determining resistance to root knot nematodes. If inoculum rate is too low, susceptible cultigens may have few root galls and appear to be resistant. On the other hand, if inoculum rate is too high, resistant cultigens may have root galls and appear to be susceptible (2).
Methods. A greenhouse study was conducted to determine the optimum egg concentration of root-knot nematodes to use when evaluating a species of Cucumis for resistance. Five egg concentrations (0, 500, 2000, 8000 and 16000 eggs/pot) of two root knot nematodes (M. incognita r. 3 and M. javanica) were used to determine optimum egg concentration for evaluating C. sativus ‘Sumter’ and C. metuliferus PI 482452 for resistance.
Plants were grown from seed, with two plants per 100-mm clay pot. The test was replicated three times. Plants contained within a pot were inoculated 2 weeks after planting with 1 of the 5 egg concentrations. Inoculum was prepared using the technique developed by Hussey and Barker (3).
Plants were rated 8 weeks after planting (6 weeks after inoculation) using the gall index system. The gall index system ranges from 0 to 100 and indicates the percentage of a root system that is galled by root knot nematodes (1).
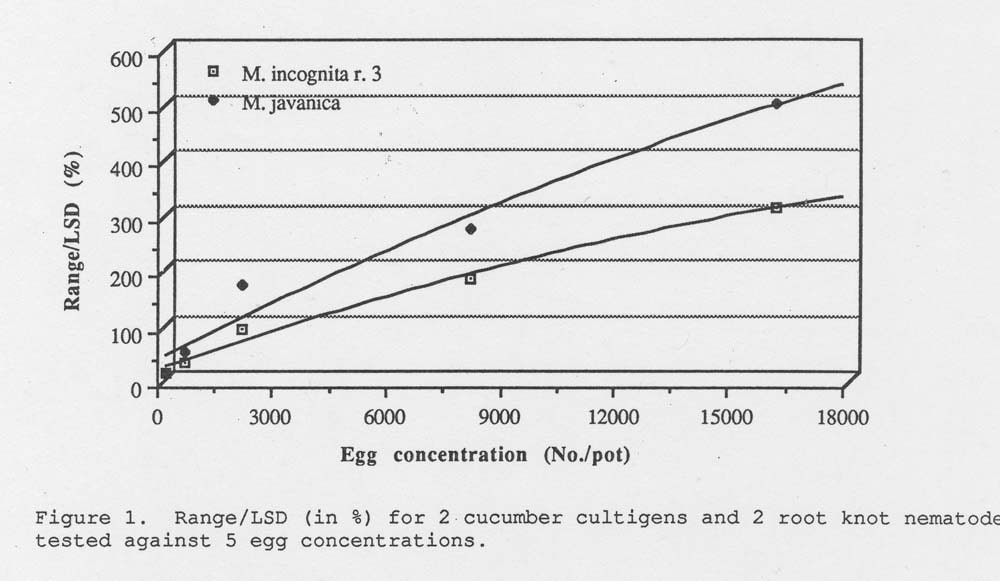
Results. The range of gall index between the 2 cucumber lines was measured for each egg concentration and root knot nematode species. This range was then divided by the LSD and converted to a percentage (Table 1). These percentages are linear for each root knot nematode tested (Fig. 1). The optimum concentration to use would be that concentration just before the curve reaches a plateau. However, in our experiment we did not include an egg concentration high enough to show the optimum.
In conclusion, a short duration test (such as this 8-week test) should use at least 16000 eggs/pot. A long duration test (such as the 12-week tests we use now), the 16000 eggs/pot level would probably be excessive. We have settled on a concentration of 8000 eggs/pot for the long duration test.
Table 1. Gall index range/LSD (as %) between cucumber cultigens for 2 nematodes and 5 egg concentration.z
Egg |
Sumter |
PI 482452 |
(%) Range/LSD |
|||
| concentration | Mi3 | Mj | Mi3 | Mj | Mi3 | Mj |
| 0 | 2 | 2 | 1 | 1 | 10 | 10 |
| 500 | 6 | 11 | 3 | 6 | 30 | 50 |
| 2000 | 17 | 26 | 8 | 9 | 90 | 170 |
| 8000 | 38 | 53 | 20 | 26 | 180 | 270 |
| 16000 | 59 | 73 | 28 | 23 | 310 | 500 |
| LSD (5%) for row-column comparisons of means | 10 | |||||
zData are means of 3 replications of 2 plants each. Mi3 – M. incognita r. 3 and Mj = M. javanica. Gall index represents percentage of root system that is galled by root knot nematodes.
Figure 1. Range /LSD (in %) for 2 cucumber cultigens and 2 root knot nematodes tested against 5 egg concentrations.
Literature Cited
- Barker, K. R. 1978. Determining nematode population responses to control agents. In (E. I. Zehr, ed.) Methods for evaluating plant fungicides, nematicides and bactericides. Amer. Phytopathol. Soc., St. Paul, Minnesota, pp. 114-125.
- Hartmann, R. W. 1976. Breeding for nematode resistance in vegetables. SABRAO J. 8: 1-10.
- Hussey, R. S. and K. R. Barker. 1973. A comparison of methods of collecting inocula of Meloidogyne spp., including a new technique. Plant Dis. Rptr. 12: 1025-1028.