Cucurbit Genetics Cooperative Report 17:111-115 (article 33) 1994
X.P. Zhang, B.B. Rhodes and J.W. Adelberg
Department of Horticulture, E-142 Poole Agricultural Center, Clemson University, Clemson, SC 29634-0375
Genetic transformation and somaclonal variation can be efficient alternatives to traditional breeding techniques for varietal improvement. However, an efficient regeneration system is essential for in vitro techniques to be used in cucurbit crops (4, 7).
Adventitious shoot regeneration from cotyledons and hypocotyls of watermelon seedlings was first reported by Srivastava et al. (1989) who determined that cotyledons were more regenerative than hypocotyls. The effects of growth regulators, genotypes, explant age and explant location in the determination of adventitious shoot organogenesis were previously documented in diploids (2, 3) and more recently in triploids (2). High frequency shoot regeneration was observed from 5-day-old cotyledons of diploid cultivars cultured on Mirashige and Skoog (MS) medium with 22,19 μ MIAA (3) or 5-10 μ M BA (2). Two growth regulator combinations, 10 μ M BA alone (1) gave high frequency shoot organogenesis.
Adventitious shoot organogenesis studies in watermelon have been conducted using cotyledons from mature seeds. Cotyledons from immature seeds can be another useful explant source for adventitious shoot regeneration (1). The cotyledons of immature seeds taken from a disinfected fruit tend to be bacteria-free, obviating the need for sterilization of explant tissue. Immature cotyledons may be more responsive to in vitro regulation than mature cotyledons because of their active physiological condition. The watermelon cotyledon is large enough to be easily sectioned. Knowledge of the location of responsive regions for regeneration can be very useful for explant preparations in somaclonal variation and transformation studies. This research explores the effect of the inclusion of NAA, age, genotype, location and size of explants on shoot regeneration from immature watermelon cotyledons.
Methods. Immature fruits from self-pollinated flowers were harvested from greenhouse grown plants during the spring-summer and fall-winter of 1991. The fruit were surface-disinfested with 95% ethanol, flamed under a laminar flow hood and sliced open. Fruit development in the greenhouse took about 35 days for the spring crop and 40 days to mature for the fall crop. Immature seeds were removed from the fruit and the developing cotyledons were excised. Either entire (minus a small segment of tissue near the apical end of the embryo to avoid the confounded factor of axillary shoot formation) cotyledons or section of cotyledons (Fig. 1) were used as explants. The cotyledon tissue was placed on the medium in abaxial orientation. MS (8) medium was prepared and supplemented according to Wilkins (1985) with 100 mg/l myo-inositol, 2 mg/l glycine, 0.2 mg/l thiamine HCl, 0.5 mg/l pyroxidine, 0.5 mg/l nicotinic acid, 7 g/l agar (Agar-Agar, Gum Agar; Catalog no. A 1296, SIGMA Chemical Co., St. Louis, MO), 30 g/l sucrose, and 10 μ M BA with \or without 1 μ M NAA. Medium pH was adjusted to 5.7 before autoclaving. Because of the limitation of available immature embryos, eight to 16 explants per vessel were cultured on 50 ml medium in Magenta GA 7 vessels (Sigma) under cool white fluorescent lamps that provided a photosynthetic flux density of 35 μ mol m-2 sec-1 for a 16 hr period at 25 + 2 ˚ C.
Shoot regeneration was compared from immature cotyledons cultured on medium with 10μ MBA + 1 μM NAA (1.10). One hundred twenty and 104 entire cotyledons dissected from seeds of G17AB 17 days after pollination in June were cultured on 10 μM BA and 10 μ M NAA medium, respectively. The percentage of cotyledons that callused before producing shoot beds (i.e., the shoots before elongation) was recorded. After 34 days, the number of cotyledons that regenerated one or more shoot buds was recorded.
Cotyledons from immature seeds collected 14, 18 and 20 days after pollination in June were evaluated for shoot regeneration competence. The genotype G17AB was used as above with 10 μM BA. At least four replications of 8 whole cotyledonary explants from each age were cultured on MS medium. The number of cotyledons that regenerated one or more shoots were recorded 35 days after culture initiation.
To determine the effect of genotype on shoot regeneration, immature fruit from8 inbred diploid genotypes were harvested 20 days after pollination in November. Lines JXP-91, G17AB, B-91, DRE, and 91.7.4 were our breeding program. Commercial cultigens were ‘Yellow Sweet’, ‘AU-Producer’, and ‘Mickeylee’. Entire cotyledons (32-127) from each genotype were cultured on medium with 10 μM BA for 35 days and scored for the presence of shoot bud regeneration.
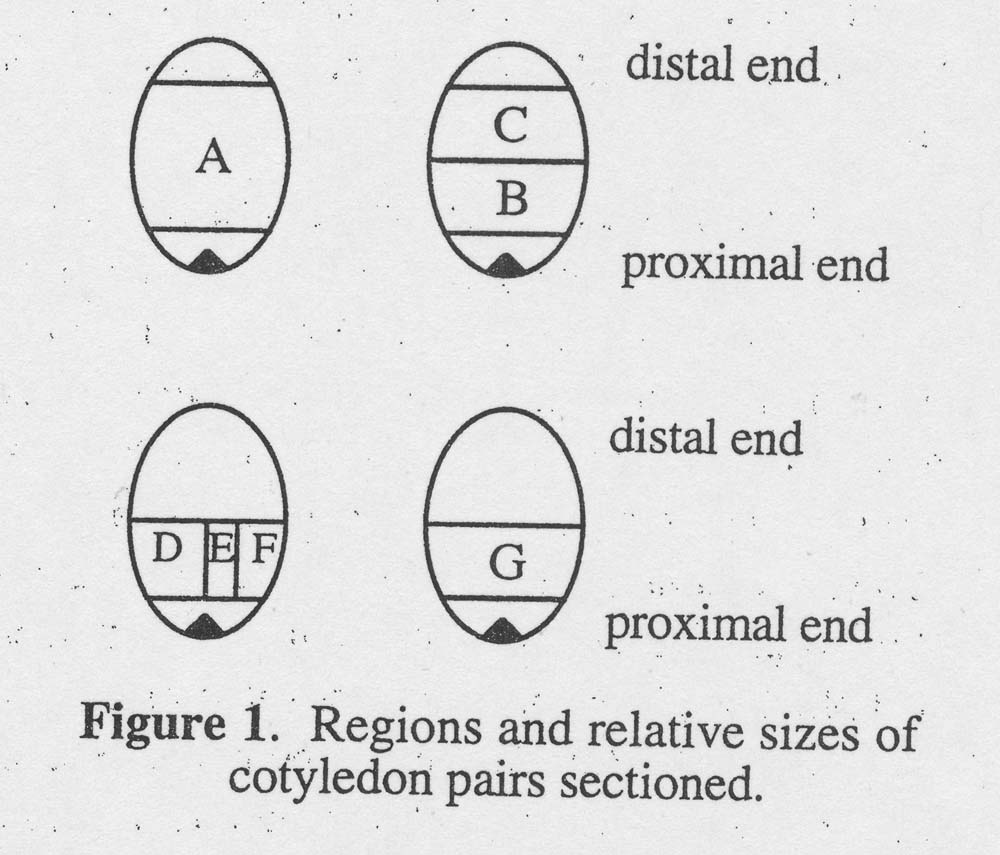
Genotype JXP-91, not different from genotype G17AB in previous testing, was used to determine the effect of location of the cotyledonary explant on shoot regeneration. Immature cotyledons were dissected (see Fig. 1) 28 days after pollination because embryo development was slow at low temperature (in December). Explant A was a large block of one cotyledon comprising all buy a small segment of tissue near the apical end of the embryo and a small segment of tissue at the distal tip of the cotyledon. The other cotyledon from the same immature embryo was cut the same way as A but then cross-sectioned into two equal portions: B (proximal portion) and C (distal portion). To obtain D, E, and F, a longitudinal cut was made to bisect the proximal block into two equal portions. One portion was bisected again to produce explants E and F. E was the center portion of the proximal portion of one cotyledon. G was taken from the same location as B, but the rest of the cotyledon was discarded. Forty-eight explants from each location were cultured on medium with 10 μ M BA. The number of explants with shoot buds was recorded after 35 days in vitro. To promote further shoot development, buds regenerated from six explants of each type were transferred to medium with 5 μM BA for 14 days.
Figure 1. Regions and relative sizes of cotyledon pairs sectioned.
To obtain plantlets, shoot buds from cotyledons on medium with 10 μM BA were transferred to medium with 5 uM IBA for 12 days. These plantlets were planted into Todd Planter Flats (Catalog No. 14-2574, A. H. Hummert Seed Co., St. Louis, MO) containing pre-moistened commercial soilless potting mix (Fafard Superfine, Fafard, Inc., Anderson, SC) and placed under mist in the greenhouse. Shoots without roots were rooted with Hormodin #1 (Merck& Co., Rahway, NJ).
The shoot regeneration frequency data (percentage) were subjected to an arcsin √ y transformation and the number of shoots per explant data were subjected to a square root transformation before the analysis of variance. An LSD was performed to determine significant differences with the analysis of variance was significant.
Results. Immature cotyledons of G17AB cultured on media with 10 μM NAA (60% and 38%, respectively). Although the apparent increase of callus on NAA was not statistically significant (77% versus 64%), auxin (NAA and IAA) increased callus formation and inhibited watermelon shoot regeneration from mature cotyledons in studies by Srivastava et al. (1989) and Compton and Gray (1993). Because of the negative effect of auxin on watermelon shoot regeneration, medium with BA alone was used for future experiments.
The effect of explant age on shoot regeneration from cotyledons excised 14-20 days after pollination was not significant in the range tested. The age of the immature cotyledon explants was not as critical as the age of cotyledons from mature seeds for optimal shoot regeneration (2, 3). The differential response may be related to the developmental difference of explant tissue from immature and mature cotyledons. The fate of cell differentiation and development in the immature cotyledon may be less determined than in the mature cotyledon, and therefore the cells in the immature cotyledon might be regulated in vitro over a longer period. It would be enlightening to compare shoot regeneration from the immature versus the mature cotyledon. Cotyledons from 20-day-old (summer) or 28-day-old (winter) embryos were chosen for future experiments because dissection of cotyledons from younger embryos was difficult.
More explants from genotypes JXP-91, G17AB and B1-91 regenerated more shoots than the other genotypes tested (Table 1). Shoot bud initiation was observed one week later for ‘Mickylee’ than the other genotypes. Genotypic differences for adventitious shoot regeneration in watermelon were previously observed using mature cotyledons (3, 2). We noted that genotypes that were difficult to regenerate either produced fewer shoots (DRE and 91.74) or callused profusely (‘Mickeylee’). In fact, more recent observations not reported here indicate that genotype not only affects shoot regeneration but also rooting and acclimatization.
Cotyledon sections, explant B. D, G and E, proximal to the cotyledonary node regenerated shoots more often and produced more shoots than cotyledon sections, explant C, from distal regions (Table 2). The excised cotyledon, explant A, produced 14 shoots per explant. When twin cotyledons were bisected, distal section,explant C, produced only 3 shoots per explant whereas the proximal section, explant B, produced 19 shoots per explant. In a parallel experiment, proximal section G (similar to B) yielded 21 shoots per explant, not different from explant B. When twin cotyledon sections were dissected into sections D, E and F, 22, 18 and 8 shoots per explant were produced, respectively, from the same quantity of tissue. Dividing explant G into D, E and F with two cuts resulted in 2.3 times as many regenerants as produced by explant G. Bisection of explant D, into E and F, yielded 11.6 times as many shoots as D alone. Within proximal portions, explants containing the midrib of cotyledons regenerated more frequently than explants from marginal portions. When explant G was bisected into D, E and F, the interior portion E always produced shoots whereas only 69% of portion F explants regenerated shoots.
The cells capable of shoot organogenesis in immature watermelon cotyledons were distributed mainly in the proximal region, especially the portion along the cotyledon midrib where the vascular system is located. Shoot buds were more uniform from proximal and interior portions than distal and exterior portions. Our results support the similar observations reported in cucumber (5), melon (6) and mature watermelon cotyledons (2).
Shoots and roots developed 12 days after buds were transferred to medium with 5 uM IBA. Depending on the genotype, 7% to 63% of the shoots produced roots. Shoots (94%), with or without roots, developed into transplants in 5 weeks in the greenhouse. More than 1000 plants regenerated from the 8 genotypes (Table 1 survived transplanting in the field. Plants from tissue culture produced fruit comparable in size to fruit from plants from seed. The plants appeared uniform and true-to-type. However, variants in ploidy level, suggested by pollen size and leaf character, and chlorophyll deficiency were observed. The occurrence of tetraploids was rare. Tetraploid regenerants were observed from G17AB (2.200), JXP-91 (5/241), B1-91 (1/387) and ‘Mickeylee’ (2/133). Two regenerants from B1-91 were chimeric, and chlorophyll deficiency was observed on the majority of leaves. No fruits were obtained from the vine with chlorophyll deficiency because of delayed female flower occurrence on those vines and lack of synchronous male flowers. Albino shoots were regenerated from G17AB in 1992 and the plantlets did not survive in the greenhouse. The only somaclonal variants obtained that could be maintained in this research were tetraploids. Some of the self-pollinated regenerants from G17AB, JXP-91, B1-91 and “Au-Producer’ were evaluated in the field in 1993. There was no variation observed in their progeny populations.
This research illustrated that the immature cotyledon explant was the source of high frequency shoot regeneration in watermelon, and the immature cotyledon from a disinfected fruit facilitated clean explant tissue. The effects of genotype and explant location should be considered for high frequency shoot regeneration. Our experiments on effect of cotyledon explant location on shoot regeneration provided useful information for precise selection of explant tissue, and these results can be applied to either genetic transformation experiments of tetraploid regeneration. Based on this study, an in vitro system for efficient tetraploid watermelon regeneration has been developed in our laboratory.
Table 1. Shoot regeneration from immature watermelon cotyledons¹ of different genotypes.
Genotype |
Total explants |
% Explants with shoot buds² |
| JXP-91 | 127 | 84 a3 |
| G17AB | 124 | 75 a |
| B1-91 | 102 | 61 ab |
| Yellow Sweet | 32 | 50 bc |
| Au-Producer | 32 | 34 bcd |
| DRE | 57 | 26 cd |
| 91.7.4 | 96 | 20 d |
| Mickeylee | 112 | 19 d |
1All cotyledons were taken from immature seeds 20 days after pollination.
2Data were collected 35 days after culture initiation.
3Denote differences at the 0.01 level of significence using the LSD test.
Table 2. Shoot regeneration from sections of immature watermelon cotyledon tissue1
Region2 |
Total explants |
% Explants with shoot buds |
Shoot buds per Explant4 |
| E | 48 | 100a3 | 17.8 a |
| G | 48 | 93.8 ab | 21.3 a |
| D | 48 | 87.5 ab | 21.7 a |
| B | 48 | 87.5 bc | 19.2 a |
| A | 48 | 77.1 bc | 14,0 a |
| F | 48 | 68.8 cd | 7.5 ab |
| C | 48 | 52.1 d | 3.2 b |
1Cotyledons were JXP-91 seeds 28 days after pollination.
2Refer to Figure 1.
3Denote significant differences at 0.01 confidence level using the LSD test.
4Based on six explants with shoots.
Literature Cited
- Adelberg, J.W. and B.B. Rhodes. 1989. Micropropagation from zygotic tissues of watermelon. In: C.E. Thomas, Ed. Proceedings Cucurbitaceae 89: Evaluation and Enhancement of Cucurbit Germplasm. USDA/ARS. Charleston,SC, pp 110-112.
- Compton, M.E. and D.J. Gray. 1993. Shoot organogenesis and plant regeneration from cotyledons of diploid, triploid, and tetraploid watermelon. J. Amer. Soc. Hort. Sci: 118-151-157.
- Dong, J.Z. and S.R. Jia. 1991. High efficiency plant regeneration from cotyledons of watermelon (Citrullus vulgaris Schrad.). Plant Cell Reports 9:559-562.
- Fang, G. and R. Grumet. 1990. Agrobacterium tumefacians mediated transformation and regeneration of muskmelon plants. Plant Cell Reports 9:160-164.
- Gambley, R.L. and W.A. Dott. 1990. An in vitro technique for the production de novo of multiple shoots in cotyledon explants of cucumber (Cucumber sativus L.). Plant Cell Tissue Organ cult. 20:177-183.
- Leshem, B. 1989. Polarity and response regions for regeneration in the cultured melon cotyledon. J. Plant Physiol. 135:237-239.
- Moreno, V. and L.A. Roig. 1990. Somaclonal variation in cucurbits. In: Y.P.S. Bajaj,. ed. Somaclonal Variation in Crop Improvement 1, Biotechnology in Agriculture and Forestry, Vol. 11, Springer-Verlag, Berlin Heidelberg, pp 435-464.
- Murashige, T. and R. Skoog. 1962. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 15:473-479.
- Srivastava, D.R., V.M. Andrianov and E.S. Piruzian. 1989. Tissue culture and plant regeneration of watermelon (Citrullus vulgaris Schrad. cv. Melitopolski). Plant Cell Reports 8:300-302.
- Wilkins, M. 1985. Micropropagation of two triploid hybrids of Citrullus lanatus (Thunb.) Matsumura and Nakai. M.S. Thesis, Clemson Univ. Clemson, SC.